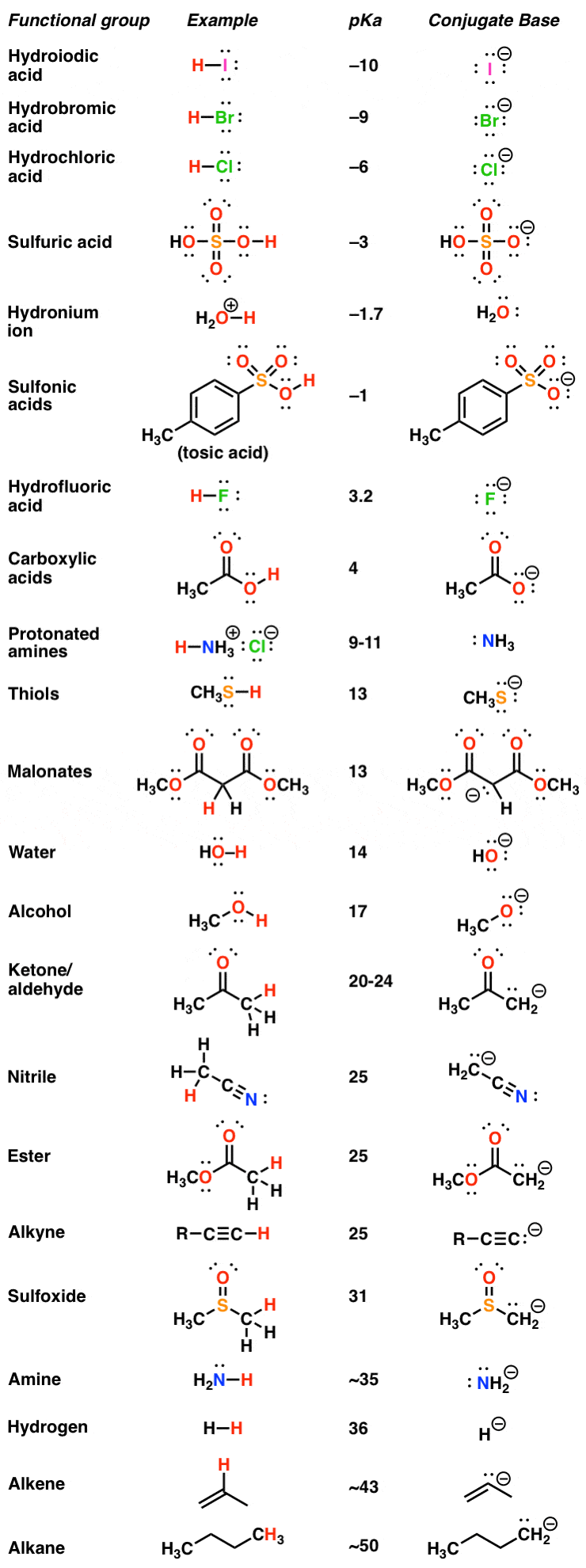
Table Of Acids And Bases . Strong acids are listed at the top left hand corner of the table and have ka values >1 2. An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a. There aren’t very many, so it’s a good idea to memorize them, if you can. Use this acids and bases chart to find the relative strength of the most common acids and bases. Acid with values less than one are considered. The word acid comes from a latin word ‘acere’ which means. Explain the ph scale, and convert ph and concentration of. Acids and bases are popular chemicals which interact with each other resulting in the formation of salt and water. This is a list of the strong acids and strong bases. Acids and bases are two types of compounds that readily react with one another. The earliest definition of acids and bases is arrhenius's definition which states that: Acids are substances that donate protons (h⁺ ions) or accept electron pairs. Give the names and formulas of some strong acids and bases.
from www.masterorganicchemistry.com
Use this acids and bases chart to find the relative strength of the most common acids and bases. Acids and bases are two types of compounds that readily react with one another. Explain the ph scale, and convert ph and concentration of. Acid with values less than one are considered. Strong acids are listed at the top left hand corner of the table and have ka values >1 2. This is a list of the strong acids and strong bases. An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a. Acids are substances that donate protons (h⁺ ions) or accept electron pairs. Give the names and formulas of some strong acids and bases. Acids and bases are popular chemicals which interact with each other resulting in the formation of salt and water.
AcidBase Reactions Introducing Ka and pKa Master Organic Chemistry
Table Of Acids And Bases Acids and bases are two types of compounds that readily react with one another. Acids are substances that donate protons (h⁺ ions) or accept electron pairs. This is a list of the strong acids and strong bases. The word acid comes from a latin word ‘acere’ which means. Use this acids and bases chart to find the relative strength of the most common acids and bases. Acids and bases are popular chemicals which interact with each other resulting in the formation of salt and water. Give the names and formulas of some strong acids and bases. Acid with values less than one are considered. The earliest definition of acids and bases is arrhenius's definition which states that: Acids and bases are two types of compounds that readily react with one another. Strong acids are listed at the top left hand corner of the table and have ka values >1 2. Explain the ph scale, and convert ph and concentration of. An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a. There aren’t very many, so it’s a good idea to memorize them, if you can.
From www.organicchemistrytutor.com
AcidBase Equilibrium Part 1 How to Use the pKa Table — Organic Table Of Acids And Bases An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a. Acids and bases are two types of compounds that readily react with one another. Give the names and formulas of some strong acids and bases. The word acid comes from a latin word ‘acere’ which means. There aren’t very many,. Table Of Acids And Bases.
From www.studypk.com
List of Strong Acids & Bases in Order StudyPK Table Of Acids And Bases There aren’t very many, so it’s a good idea to memorize them, if you can. Explain the ph scale, and convert ph and concentration of. The word acid comes from a latin word ‘acere’ which means. Give the names and formulas of some strong acids and bases. Strong acids are listed at the top left hand corner of the table. Table Of Acids And Bases.
From byjus.com
pH Of Acids And Bases Calculate pH Value Chemistry Byju's Table Of Acids And Bases Strong acids are listed at the top left hand corner of the table and have ka values >1 2. Use this acids and bases chart to find the relative strength of the most common acids and bases. Acid with values less than one are considered. Acids and bases are two types of compounds that readily react with one another. Acids. Table Of Acids And Bases.
From acidbaseindicator.weebly.com
Properties of Acids and Bases Table Of Acids And Bases The word acid comes from a latin word ‘acere’ which means. Acids and bases are popular chemicals which interact with each other resulting in the formation of salt and water. Give the names and formulas of some strong acids and bases. An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is. Table Of Acids And Bases.
From www.vrogue.co
Acid And Base Chart Table Of Acids Bases vrogue.co Table Of Acids And Bases Acid with values less than one are considered. Give the names and formulas of some strong acids and bases. Acids and bases are popular chemicals which interact with each other resulting in the formation of salt and water. Strong acids are listed at the top left hand corner of the table and have ka values >1 2. The earliest definition. Table Of Acids And Bases.
From dashamlav.com
Acid vs Base Table of Differences between Acid and Base Table Of Acids And Bases Explain the ph scale, and convert ph and concentration of. Acids and bases are two types of compounds that readily react with one another. The word acid comes from a latin word ‘acere’ which means. An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a. The earliest definition of acids. Table Of Acids And Bases.
From www.worksheetsplanet.com
Acid and Bases Differences Table Of Acids And Bases Acids and bases are two types of compounds that readily react with one another. Acid with values less than one are considered. This is a list of the strong acids and strong bases. The word acid comes from a latin word ‘acere’ which means. An acid is a substance that forms hydrogen ions h + when dissolved in water, and. Table Of Acids And Bases.
From www.teachoo.com
Strength of Acids and Bases (How to find it?) Chemistry Teachoo Table Of Acids And Bases Acids and bases are popular chemicals which interact with each other resulting in the formation of salt and water. The earliest definition of acids and bases is arrhenius's definition which states that: Acids and bases are two types of compounds that readily react with one another. Give the names and formulas of some strong acids and bases. This is a. Table Of Acids And Bases.
From www.preclaboratories.com
Back to Basics Acids, Bases & the pH Scale Precision Laboratories Table Of Acids And Bases Give the names and formulas of some strong acids and bases. Acids and bases are popular chemicals which interact with each other resulting in the formation of salt and water. Acid with values less than one are considered. The earliest definition of acids and bases is arrhenius's definition which states that: The word acid comes from a latin word ‘acere’. Table Of Acids And Bases.
From www.vrogue.co
Acid Base Strength Charts For Chemistry vrogue.co Table Of Acids And Bases Acid with values less than one are considered. There aren’t very many, so it’s a good idea to memorize them, if you can. Acids and bases are popular chemicals which interact with each other resulting in the formation of salt and water. Acids are substances that donate protons (h⁺ ions) or accept electron pairs. The earliest definition of acids and. Table Of Acids And Bases.
From narodnatribuna.info
Common Examples Of Acids And Bases A Great Explanation Table Of Acids And Bases There aren’t very many, so it’s a good idea to memorize them, if you can. Acid with values less than one are considered. Explain the ph scale, and convert ph and concentration of. Acids and bases are two types of compounds that readily react with one another. The earliest definition of acids and bases is arrhenius's definition which states that:. Table Of Acids And Bases.
From secondaryscience4all.wordpress.com
Acids and bases Secondary Science 4 All Table Of Acids And Bases Strong acids are listed at the top left hand corner of the table and have ka values >1 2. Use this acids and bases chart to find the relative strength of the most common acids and bases. Acids are substances that donate protons (h⁺ ions) or accept electron pairs. There aren’t very many, so it’s a good idea to memorize. Table Of Acids And Bases.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo Table Of Acids And Bases The word acid comes from a latin word ‘acere’ which means. Give the names and formulas of some strong acids and bases. Acids and bases are two types of compounds that readily react with one another. The earliest definition of acids and bases is arrhenius's definition which states that: Acid with values less than one are considered. Strong acids are. Table Of Acids And Bases.
From pediaa.com
Difference Between Acid and Base Table Of Acids And Bases Acids and bases are popular chemicals which interact with each other resulting in the formation of salt and water. Explain the ph scale, and convert ph and concentration of. Use this acids and bases chart to find the relative strength of the most common acids and bases. An acid is a substance that forms hydrogen ions h + when dissolved. Table Of Acids And Bases.
From www.flinnsci.ca
AcidBase Strength Charts for Chemistry Table Of Acids And Bases The earliest definition of acids and bases is arrhenius's definition which states that: Strong acids are listed at the top left hand corner of the table and have ka values >1 2. Acids and bases are two types of compounds that readily react with one another. Acids and bases are popular chemicals which interact with each other resulting in the. Table Of Acids And Bases.
From www.flinnsci.ca
AcidBase Strength Charts for Chemistry Table Of Acids And Bases Acid with values less than one are considered. The earliest definition of acids and bases is arrhenius's definition which states that: Use this acids and bases chart to find the relative strength of the most common acids and bases. Acids are substances that donate protons (h⁺ ions) or accept electron pairs. There aren’t very many, so it’s a good idea. Table Of Acids And Bases.
From sciencenotes.org
AcidBase Chemistry Table Of Acids And Bases Explain the ph scale, and convert ph and concentration of. Use this acids and bases chart to find the relative strength of the most common acids and bases. An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a. The earliest definition of acids and bases is arrhenius's definition which states. Table Of Acids And Bases.
From courses.lumenlearning.com
BrønstedLowry Acids and Bases MCC Organic Chemistry Table Of Acids And Bases The earliest definition of acids and bases is arrhenius's definition which states that: An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a. This is a list of the strong acids and strong bases. Strong acids are listed at the top left hand corner of the table and have ka. Table Of Acids And Bases.
From askstims.weebly.com
Periodic Table Acids and bases Table Of Acids And Bases Give the names and formulas of some strong acids and bases. This is a list of the strong acids and strong bases. The earliest definition of acids and bases is arrhenius's definition which states that: The word acid comes from a latin word ‘acere’ which means. There aren’t very many, so it’s a good idea to memorize them, if you. Table Of Acids And Bases.
From learningmagicprevision.z13.web.core.windows.net
Acids Bases And The Ph Scale Table Of Acids And Bases Explain the ph scale, and convert ph and concentration of. Use this acids and bases chart to find the relative strength of the most common acids and bases. Give the names and formulas of some strong acids and bases. Acids are substances that donate protons (h⁺ ions) or accept electron pairs. An acid is a substance that forms hydrogen ions. Table Of Acids And Bases.
From www.masterorganicchemistry.com
AcidBase Reactions Introducing Ka and pKa Master Organic Chemistry Table Of Acids And Bases There aren’t very many, so it’s a good idea to memorize them, if you can. Acids are substances that donate protons (h⁺ ions) or accept electron pairs. The word acid comes from a latin word ‘acere’ which means. Strong acids are listed at the top left hand corner of the table and have ka values >1 2. Acid with values. Table Of Acids And Bases.
From classlibjeske.z21.web.core.windows.net
List The Properties Of Acids And Bases Table Of Acids And Bases Acids are substances that donate protons (h⁺ ions) or accept electron pairs. Give the names and formulas of some strong acids and bases. There aren’t very many, so it’s a good idea to memorize them, if you can. An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a. The earliest. Table Of Acids And Bases.
From courses.lumenlearning.com
Relative Strengths of Acids and Bases Chemistry Table Of Acids And Bases Give the names and formulas of some strong acids and bases. The earliest definition of acids and bases is arrhenius's definition which states that: An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a. Acid with values less than one are considered. Use this acids and bases chart to find. Table Of Acids And Bases.
From classmagicwong101.z13.web.core.windows.net
Different Theories Of Acid And Base Table Of Acids And Bases Use this acids and bases chart to find the relative strength of the most common acids and bases. Acids and bases are two types of compounds that readily react with one another. Acids are substances that donate protons (h⁺ ions) or accept electron pairs. Acids and bases are popular chemicals which interact with each other resulting in the formation of. Table Of Acids And Bases.
From kidspressmagazine.com
Acids and Bases Table Of Acids And Bases Strong acids are listed at the top left hand corner of the table and have ka values >1 2. This is a list of the strong acids and strong bases. Acids are substances that donate protons (h⁺ ions) or accept electron pairs. The earliest definition of acids and bases is arrhenius's definition which states that: An acid is a substance. Table Of Acids And Bases.
From www.priyamstudycentre.com
Acids and Bases Definition, Concept, Theory, Examples Table Of Acids And Bases Acids and bases are popular chemicals which interact with each other resulting in the formation of salt and water. Strong acids are listed at the top left hand corner of the table and have ka values >1 2. The earliest definition of acids and bases is arrhenius's definition which states that: Use this acids and bases chart to find the. Table Of Acids And Bases.
From courses.lumenlearning.com
Relative Strengths of Acids and Bases General Chemistry Table Of Acids And Bases This is a list of the strong acids and strong bases. Acid with values less than one are considered. The earliest definition of acids and bases is arrhenius's definition which states that: Strong acids are listed at the top left hand corner of the table and have ka values >1 2. Explain the ph scale, and convert ph and concentration. Table Of Acids And Bases.
From jonathanhutchinson.z21.web.core.windows.net
Acid And Base Chart Table Of Acids And Bases The earliest definition of acids and bases is arrhenius's definition which states that: Use this acids and bases chart to find the relative strength of the most common acids and bases. Acids are substances that donate protons (h⁺ ions) or accept electron pairs. Acids and bases are two types of compounds that readily react with one another. Strong acids are. Table Of Acids And Bases.
From studylib.net
Acid and Base Summary 1 Table Of Acids And Bases Use this acids and bases chart to find the relative strength of the most common acids and bases. An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a. There aren’t very many, so it’s a good idea to memorize them, if you can. Acid with values less than one are. Table Of Acids And Bases.
From coqobyvipev.carriagehouseautoresto.com
An Overview Of Common Acids And Bases Table Of Acids And Bases Acids and bases are two types of compounds that readily react with one another. There aren’t very many, so it’s a good idea to memorize them, if you can. Explain the ph scale, and convert ph and concentration of. Acid with values less than one are considered. Acids are substances that donate protons (h⁺ ions) or accept electron pairs. The. Table Of Acids And Bases.
From lessondbenduringly.z22.web.core.windows.net
List The Properties Of Acids And Bases Table Of Acids And Bases There aren’t very many, so it’s a good idea to memorize them, if you can. Acid with values less than one are considered. Explain the ph scale, and convert ph and concentration of. Acids are substances that donate protons (h⁺ ions) or accept electron pairs. Use this acids and bases chart to find the relative strength of the most common. Table Of Acids And Bases.
From www.scitk.org
Hard and Soft Acids and Bases (HSAB) Table Of Acids And Bases Acid with values less than one are considered. Acids and bases are two types of compounds that readily react with one another. Strong acids are listed at the top left hand corner of the table and have ka values >1 2. There aren’t very many, so it’s a good idea to memorize them, if you can. This is a list. Table Of Acids And Bases.
From www.sigmaaldrich.com
Acid and Base Chart — Table of Acids & Bases Table Of Acids And Bases Give the names and formulas of some strong acids and bases. This is a list of the strong acids and strong bases. Use this acids and bases chart to find the relative strength of the most common acids and bases. An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a.. Table Of Acids And Bases.
From issr.edu.kh
pKa Values and strengths of Acids and Bases Table Of Acids And Bases The earliest definition of acids and bases is arrhenius's definition which states that: Acids are substances that donate protons (h⁺ ions) or accept electron pairs. Explain the ph scale, and convert ph and concentration of. Acid with values less than one are considered. There aren’t very many, so it’s a good idea to memorize them, if you can. This is. Table Of Acids And Bases.
From www.vrogue.co
Chemistry Naming Acids Rules And Practice vrogue.co Table Of Acids And Bases Explain the ph scale, and convert ph and concentration of. There aren’t very many, so it’s a good idea to memorize them, if you can. The earliest definition of acids and bases is arrhenius's definition which states that: Use this acids and bases chart to find the relative strength of the most common acids and bases. Give the names and. Table Of Acids And Bases.