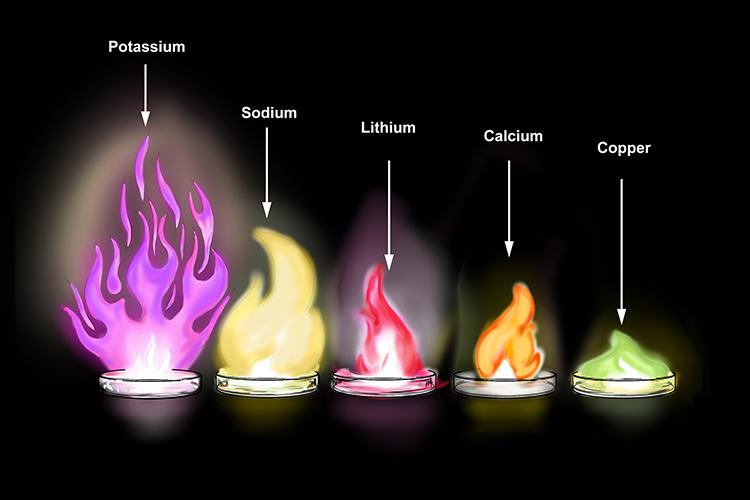
Do Metals Burn In Oxygen . When heated, lithium, sodium, potassium, rubidium, and cesium ignite through combustion reactions with oxygen. Beryllium, magnesium and calcium don't form peroxides when heated in oxygen, but strontium and barium do. Lithium, the first metal in group 1, reacts with oxygen to form li 2 o and burns with a red flame. Potassium and sodium are soft metal which are. There is an increase in the tendency to form the peroxide as you go down the group. Potassium, sodium, lithium, calcium and magnesium react with oxygen and burn in air. Why do some metals form peroxides on heating in oxygen? Many metals react with oxygen to form metal oxides. Beryllium, magnesium and calcium don't form peroxides when heated in oxygen, but strontium and barium do. Why do some metals form peroxides on heating in oxygen? The reaction of metals with air (oxygen). Metal oxides act as bases. Reactions of metals with oxygen in air. This short video shows what happens when four metals are heated in. Burning elements in air or oxygen.
from mammothmemory.net
Many metals react with oxygen to form metal oxides. Beryllium, magnesium and calcium don't form peroxides when heated in oxygen, but strontium and barium do. When heated, lithium, sodium, potassium, rubidium, and cesium ignite through combustion reactions with oxygen. Lithium, the first metal in group 1, reacts with oxygen to form li 2 o and burns with a red flame. Metal oxides act as bases. Potassium, sodium, lithium, calcium and magnesium react with oxygen and burn in air. Oxygen only makes up about 20% of air, the rest being mainly nitrogen. This short video shows what happens when four metals are heated in. There is an increase in the tendency to form the peroxide as you go down the group. Why do some metals form peroxides on heating in oxygen?
When metals are heated it reacts with oxygen to create flame
Do Metals Burn In Oxygen Why do some metals form peroxides on heating in oxygen? Beryllium, magnesium and calcium don't form peroxides when heated in oxygen, but strontium and barium do. Beryllium, magnesium and calcium don't form peroxides when heated in oxygen, but strontium and barium do. There is an increase in the tendency to form the peroxide as you go down the group. Oxygen only makes up about 20% of air, the rest being mainly nitrogen. This short video shows what happens when four metals are heated in. Why do some metals form peroxides on heating in oxygen? Why do some metals form peroxides on heating in oxygen? Burning elements in air or oxygen. The reaction of metals with air (oxygen). Potassium, sodium, lithium, calcium and magnesium react with oxygen and burn in air. Lithium, the first metal in group 1, reacts with oxygen to form li 2 o and burns with a red flame. When heated, lithium, sodium, potassium, rubidium, and cesium ignite through combustion reactions with oxygen. Potassium and sodium are soft metal which are. Reactions of metals with oxygen in air. Many metals react with oxygen to form metal oxides.
From www.slideshare.net
Reaction of metal with oxygen Do Metals Burn In Oxygen When heated, lithium, sodium, potassium, rubidium, and cesium ignite through combustion reactions with oxygen. This short video shows what happens when four metals are heated in. Many metals react with oxygen to form metal oxides. Beryllium, magnesium and calcium don't form peroxides when heated in oxygen, but strontium and barium do. Metal oxides act as bases. Potassium and sodium are. Do Metals Burn In Oxygen.
From www.slideserve.com
PPT Topic 11 PowerPoint Presentation, free download ID5285480 Do Metals Burn In Oxygen When heated, lithium, sodium, potassium, rubidium, and cesium ignite through combustion reactions with oxygen. The reaction of metals with air (oxygen). Why do some metals form peroxides on heating in oxygen? Why do some metals form peroxides on heating in oxygen? Burning elements in air or oxygen. Metal oxides act as bases. Potassium and sodium are soft metal which are.. Do Metals Burn In Oxygen.
From www.youtube.com
Reaction of Magnesium with Oxygen Gas (Burning Magnesium in Air) YouTube Do Metals Burn In Oxygen Lithium, the first metal in group 1, reacts with oxygen to form li 2 o and burns with a red flame. Why do some metals form peroxides on heating in oxygen? Reactions of metals with oxygen in air. Beryllium, magnesium and calcium don't form peroxides when heated in oxygen, but strontium and barium do. Many metals react with oxygen to. Do Metals Burn In Oxygen.
From www.slideserve.com
PPT Reacting metals with oxygen PowerPoint Presentation ID6645971 Do Metals Burn In Oxygen Beryllium, magnesium and calcium don't form peroxides when heated in oxygen, but strontium and barium do. Oxygen only makes up about 20% of air, the rest being mainly nitrogen. Why do some metals form peroxides on heating in oxygen? Reactions of metals with oxygen in air. Potassium and sodium are soft metal which are. This short video shows what happens. Do Metals Burn In Oxygen.
From www.slideserve.com
PPT Chapter 9 Chemical Reactions PowerPoint Presentation, free Do Metals Burn In Oxygen This short video shows what happens when four metals are heated in. There is an increase in the tendency to form the peroxide as you go down the group. Metal oxides act as bases. Many metals react with oxygen to form metal oxides. When heated, lithium, sodium, potassium, rubidium, and cesium ignite through combustion reactions with oxygen. The reaction of. Do Metals Burn In Oxygen.
From melscience.com
Burning in pure oxygen MEL Chemistry Do Metals Burn In Oxygen Why do some metals form peroxides on heating in oxygen? Burning elements in air or oxygen. There is an increase in the tendency to form the peroxide as you go down the group. Reactions of metals with oxygen in air. Potassium and sodium are soft metal which are. Beryllium, magnesium and calcium don't form peroxides when heated in oxygen, but. Do Metals Burn In Oxygen.
From slideplayer.com
What is made when different substances burn in oxygen ppt download Do Metals Burn In Oxygen When heated, lithium, sodium, potassium, rubidium, and cesium ignite through combustion reactions with oxygen. Why do some metals form peroxides on heating in oxygen? Burning elements in air or oxygen. Beryllium, magnesium and calcium don't form peroxides when heated in oxygen, but strontium and barium do. Lithium, the first metal in group 1, reacts with oxygen to form li 2. Do Metals Burn In Oxygen.
From www.youtube.com
💯 An Ultimate Guide to Reactions of Metals with Oxygen Reaction of Do Metals Burn In Oxygen Beryllium, magnesium and calcium don't form peroxides when heated in oxygen, but strontium and barium do. Potassium and sodium are soft metal which are. This short video shows what happens when four metals are heated in. The reaction of metals with air (oxygen). Many metals react with oxygen to form metal oxides. Oxygen only makes up about 20% of air,. Do Metals Burn In Oxygen.
From slideplayer.com
What is made when different substances burn in oxygen ppt download Do Metals Burn In Oxygen Lithium, the first metal in group 1, reacts with oxygen to form li 2 o and burns with a red flame. Potassium, sodium, lithium, calcium and magnesium react with oxygen and burn in air. Reactions of metals with oxygen in air. This short video shows what happens when four metals are heated in. The reaction of metals with air (oxygen).. Do Metals Burn In Oxygen.
From www.slideserve.com
PPT Reactions of Metals PowerPoint Presentation, free download ID Do Metals Burn In Oxygen Why do some metals form peroxides on heating in oxygen? Beryllium, magnesium and calcium don't form peroxides when heated in oxygen, but strontium and barium do. Reactions of metals with oxygen in air. Why do some metals form peroxides on heating in oxygen? Beryllium, magnesium and calcium don't form peroxides when heated in oxygen, but strontium and barium do. The. Do Metals Burn In Oxygen.
From melscience.com
Burning in pure oxygen MEL Chemistry Do Metals Burn In Oxygen Reactions of metals with oxygen in air. Lithium, the first metal in group 1, reacts with oxygen to form li 2 o and burns with a red flame. When heated, lithium, sodium, potassium, rubidium, and cesium ignite through combustion reactions with oxygen. Why do some metals form peroxides on heating in oxygen? Potassium and sodium are soft metal which are.. Do Metals Burn In Oxygen.
From dayamiaresbarrett.blogspot.com
Reaction of Metals With Oxygen DayamiaresBarrett Do Metals Burn In Oxygen Beryllium, magnesium and calcium don't form peroxides when heated in oxygen, but strontium and barium do. Potassium, sodium, lithium, calcium and magnesium react with oxygen and burn in air. Oxygen only makes up about 20% of air, the rest being mainly nitrogen. Potassium and sodium are soft metal which are. Reactions of metals with oxygen in air. Many metals react. Do Metals Burn In Oxygen.
From slideplayer.com
What is made when different substances burn in oxygen ppt download Do Metals Burn In Oxygen Beryllium, magnesium and calcium don't form peroxides when heated in oxygen, but strontium and barium do. Metal oxides act as bases. Potassium, sodium, lithium, calcium and magnesium react with oxygen and burn in air. There is an increase in the tendency to form the peroxide as you go down the group. Potassium and sodium are soft metal which are. Oxygen. Do Metals Burn In Oxygen.
From www.youtube.com
Reaction of Metals with Oxygen Burning Copper YouTube Do Metals Burn In Oxygen Why do some metals form peroxides on heating in oxygen? Potassium and sodium are soft metal which are. Potassium, sodium, lithium, calcium and magnesium react with oxygen and burn in air. When heated, lithium, sodium, potassium, rubidium, and cesium ignite through combustion reactions with oxygen. The reaction of metals with air (oxygen). Why do some metals form peroxides on heating. Do Metals Burn In Oxygen.
From www.youtube.com
Burning Sulfur in Oxygen YouTube Do Metals Burn In Oxygen Potassium and sodium are soft metal which are. Burning elements in air or oxygen. Oxygen only makes up about 20% of air, the rest being mainly nitrogen. Metal oxides act as bases. Why do some metals form peroxides on heating in oxygen? There is an increase in the tendency to form the peroxide as you go down the group. Many. Do Metals Burn In Oxygen.
From www.youtube.com
Chemistry experiment 35 Iron burning in oxygen YouTube Do Metals Burn In Oxygen When heated, lithium, sodium, potassium, rubidium, and cesium ignite through combustion reactions with oxygen. Beryllium, magnesium and calcium don't form peroxides when heated in oxygen, but strontium and barium do. Metal oxides act as bases. The reaction of metals with air (oxygen). Burning elements in air or oxygen. Oxygen only makes up about 20% of air, the rest being mainly. Do Metals Burn In Oxygen.
From www.slideserve.com
PPT Topic 11 PowerPoint Presentation, free download ID5285480 Do Metals Burn In Oxygen Burning elements in air or oxygen. Many metals react with oxygen to form metal oxides. Why do some metals form peroxides on heating in oxygen? Potassium, sodium, lithium, calcium and magnesium react with oxygen and burn in air. Reactions of metals with oxygen in air. The reaction of metals with air (oxygen). Potassium and sodium are soft metal which are.. Do Metals Burn In Oxygen.
From www.youtube.com
The reactions of metals with oxygen YouTube Do Metals Burn In Oxygen This short video shows what happens when four metals are heated in. Lithium, the first metal in group 1, reacts with oxygen to form li 2 o and burns with a red flame. Reactions of metals with oxygen in air. Potassium and sodium are soft metal which are. Potassium, sodium, lithium, calcium and magnesium react with oxygen and burn in. Do Metals Burn In Oxygen.
From slideplayer.com
Metals Oxides and NonMetal Oxides ppt download Do Metals Burn In Oxygen Metal oxides act as bases. Potassium, sodium, lithium, calcium and magnesium react with oxygen and burn in air. Reactions of metals with oxygen in air. Many metals react with oxygen to form metal oxides. Potassium and sodium are soft metal which are. When heated, lithium, sodium, potassium, rubidium, and cesium ignite through combustion reactions with oxygen. Lithium, the first metal. Do Metals Burn In Oxygen.
From www.youtube.com
Burning sodium metal in oxygen YouTube Do Metals Burn In Oxygen Metal oxides act as bases. There is an increase in the tendency to form the peroxide as you go down the group. Why do some metals form peroxides on heating in oxygen? Reactions of metals with oxygen in air. The reaction of metals with air (oxygen). Potassium and sodium are soft metal which are. When heated, lithium, sodium, potassium, rubidium,. Do Metals Burn In Oxygen.
From www.youtube.com
Iron burning in atmosphere of pure oxygen YouTube Do Metals Burn In Oxygen Reactions of metals with oxygen in air. When heated, lithium, sodium, potassium, rubidium, and cesium ignite through combustion reactions with oxygen. Many metals react with oxygen to form metal oxides. Why do some metals form peroxides on heating in oxygen? Potassium and sodium are soft metal which are. Oxygen only makes up about 20% of air, the rest being mainly. Do Metals Burn In Oxygen.
From www.youtube.com
Alkali Metals and Oxygen. See what happens when I burn alkali metals Do Metals Burn In Oxygen Reactions of metals with oxygen in air. Potassium, sodium, lithium, calcium and magnesium react with oxygen and burn in air. Oxygen only makes up about 20% of air, the rest being mainly nitrogen. Why do some metals form peroxides on heating in oxygen? Beryllium, magnesium and calcium don't form peroxides when heated in oxygen, but strontium and barium do. Potassium. Do Metals Burn In Oxygen.
From www.sciencephoto.com
Magnesium burning in oxygen Stock Image C021/1585 Science Photo Do Metals Burn In Oxygen When heated, lithium, sodium, potassium, rubidium, and cesium ignite through combustion reactions with oxygen. There is an increase in the tendency to form the peroxide as you go down the group. Many metals react with oxygen to form metal oxides. Beryllium, magnesium and calcium don't form peroxides when heated in oxygen, but strontium and barium do. Why do some metals. Do Metals Burn In Oxygen.
From www.edplace.com
Investigate How Metals React with Oxygen and Acid Worksheet EdPlace Do Metals Burn In Oxygen Lithium, the first metal in group 1, reacts with oxygen to form li 2 o and burns with a red flame. There is an increase in the tendency to form the peroxide as you go down the group. When heated, lithium, sodium, potassium, rubidium, and cesium ignite through combustion reactions with oxygen. Beryllium, magnesium and calcium don't form peroxides when. Do Metals Burn In Oxygen.
From www.youtube.com
The Reaction of Metals with Oxygen YouTube Do Metals Burn In Oxygen Beryllium, magnesium and calcium don't form peroxides when heated in oxygen, but strontium and barium do. Potassium, sodium, lithium, calcium and magnesium react with oxygen and burn in air. When heated, lithium, sodium, potassium, rubidium, and cesium ignite through combustion reactions with oxygen. Why do some metals form peroxides on heating in oxygen? Beryllium, magnesium and calcium don't form peroxides. Do Metals Burn In Oxygen.
From melscience.com
Burning in pure oxygen MEL Chemistry Do Metals Burn In Oxygen Why do some metals form peroxides on heating in oxygen? Beryllium, magnesium and calcium don't form peroxides when heated in oxygen, but strontium and barium do. Many metals react with oxygen to form metal oxides. The reaction of metals with air (oxygen). Potassium and sodium are soft metal which are. Why do some metals form peroxides on heating in oxygen?. Do Metals Burn In Oxygen.
From www.sciencephoto.com
Steel wool burning in a stream of oxygen Stock Image A510/0160 Do Metals Burn In Oxygen Many metals react with oxygen to form metal oxides. Why do some metals form peroxides on heating in oxygen? Oxygen only makes up about 20% of air, the rest being mainly nitrogen. Metal oxides act as bases. Reactions of metals with oxygen in air. Potassium and sodium are soft metal which are. Burning elements in air or oxygen. Beryllium, magnesium. Do Metals Burn In Oxygen.
From slideplayer.com
What is made when different substances burn in oxygen ppt download Do Metals Burn In Oxygen Potassium and sodium are soft metal which are. Beryllium, magnesium and calcium don't form peroxides when heated in oxygen, but strontium and barium do. Potassium, sodium, lithium, calcium and magnesium react with oxygen and burn in air. The reaction of metals with air (oxygen). Burning elements in air or oxygen. This short video shows what happens when four metals are. Do Metals Burn In Oxygen.
From mammothmemory.net
When metals are heated it reacts with oxygen to create flame Do Metals Burn In Oxygen When heated, lithium, sodium, potassium, rubidium, and cesium ignite through combustion reactions with oxygen. Beryllium, magnesium and calcium don't form peroxides when heated in oxygen, but strontium and barium do. Why do some metals form peroxides on heating in oxygen? Many metals react with oxygen to form metal oxides. Potassium and sodium are soft metal which are. There is an. Do Metals Burn In Oxygen.
From www.slideserve.com
PPT CHAPTER 3 METALS AND NON METALS PowerPoint Presentation ID598959 Do Metals Burn In Oxygen Why do some metals form peroxides on heating in oxygen? The reaction of metals with air (oxygen). Beryllium, magnesium and calcium don't form peroxides when heated in oxygen, but strontium and barium do. Potassium and sodium are soft metal which are. Oxygen only makes up about 20% of air, the rest being mainly nitrogen. Metal oxides act as bases. Why. Do Metals Burn In Oxygen.
From www.teacharesources.com
Grade 9 Metals burning in oxygen in animated PowerPoint. • Teacha! Do Metals Burn In Oxygen Many metals react with oxygen to form metal oxides. Beryllium, magnesium and calcium don't form peroxides when heated in oxygen, but strontium and barium do. There is an increase in the tendency to form the peroxide as you go down the group. Burning elements in air or oxygen. Potassium, sodium, lithium, calcium and magnesium react with oxygen and burn in. Do Metals Burn In Oxygen.
From www.scienceabc.com
What Is Oxidation? What Is An Oxidation Number? Do Metals Burn In Oxygen Why do some metals form peroxides on heating in oxygen? Lithium, the first metal in group 1, reacts with oxygen to form li 2 o and burns with a red flame. Burning elements in air or oxygen. When heated, lithium, sodium, potassium, rubidium, and cesium ignite through combustion reactions with oxygen. This short video shows what happens when four metals. Do Metals Burn In Oxygen.
From www.youtube.com
Burning metals in liquid oxygen! YouTube Do Metals Burn In Oxygen Burning elements in air or oxygen. Potassium and sodium are soft metal which are. There is an increase in the tendency to form the peroxide as you go down the group. Lithium, the first metal in group 1, reacts with oxygen to form li 2 o and burns with a red flame. Oxygen only makes up about 20% of air,. Do Metals Burn In Oxygen.
From www.teachoo.com
Reaction of Metals and Nonmetals with Oxygen Concepts Do Metals Burn In Oxygen Why do some metals form peroxides on heating in oxygen? Why do some metals form peroxides on heating in oxygen? When heated, lithium, sodium, potassium, rubidium, and cesium ignite through combustion reactions with oxygen. This short video shows what happens when four metals are heated in. The reaction of metals with air (oxygen). There is an increase in the tendency. Do Metals Burn In Oxygen.
From www.slideserve.com
PPT Reacting metals with oxygen PowerPoint Presentation, free Do Metals Burn In Oxygen There is an increase in the tendency to form the peroxide as you go down the group. Beryllium, magnesium and calcium don't form peroxides when heated in oxygen, but strontium and barium do. Metal oxides act as bases. Why do some metals form peroxides on heating in oxygen? Reactions of metals with oxygen in air. This short video shows what. Do Metals Burn In Oxygen.