What Is Rate Constant In Chemical Kinetics . The rate of a reaction is the change in concentration of either the reactant or product with respect to time. The rate therefore has units of. A rate law is an expression showing the relationship of the reaction rate to the concentrations of each reactant. Rate = k [a] 0. When we talk about the rate of a chemical reaction, what we mean is the rate at which reactants are used up, or equivalently the rate at which products are formed. Rate laws or rate equations are mathematical expressions that describe the relationship between the rate of a chemical reaction and the. The rate constant is a proportionality factor in the rate law that quantifies the speed of a chemical reaction at a given temperature. During the course of a reaction, the concentration of the reactants will decrease and.
from www.youtube.com
The rate therefore has units of. The rate constant is a proportionality factor in the rate law that quantifies the speed of a chemical reaction at a given temperature. When we talk about the rate of a chemical reaction, what we mean is the rate at which reactants are used up, or equivalently the rate at which products are formed. Rate laws or rate equations are mathematical expressions that describe the relationship between the rate of a chemical reaction and the. Rate = k [a] 0. The rate of a reaction is the change in concentration of either the reactant or product with respect to time. A rate law is an expression showing the relationship of the reaction rate to the concentrations of each reactant. During the course of a reaction, the concentration of the reactants will decrease and.
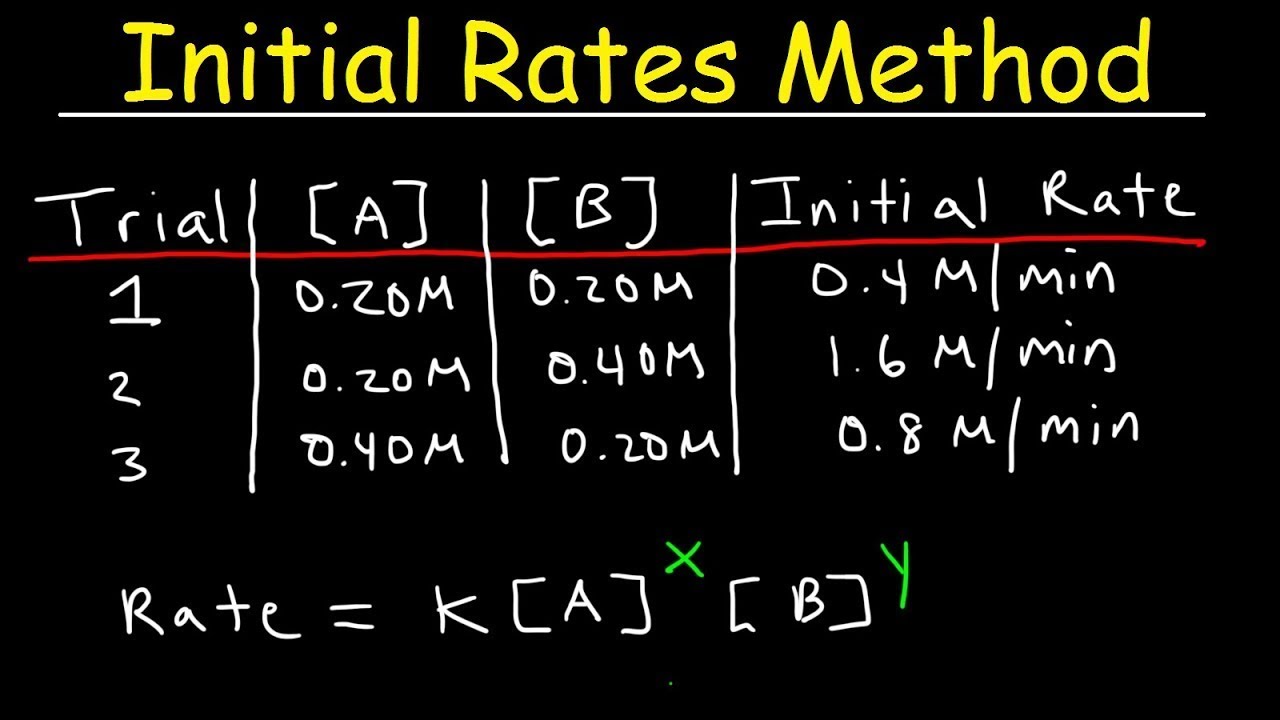
Chemical Initial Rates Method YouTube
What Is Rate Constant In Chemical Kinetics Rate laws or rate equations are mathematical expressions that describe the relationship between the rate of a chemical reaction and the. The rate constant is a proportionality factor in the rate law that quantifies the speed of a chemical reaction at a given temperature. When we talk about the rate of a chemical reaction, what we mean is the rate at which reactants are used up, or equivalently the rate at which products are formed. A rate law is an expression showing the relationship of the reaction rate to the concentrations of each reactant. Rate = k [a] 0. During the course of a reaction, the concentration of the reactants will decrease and. Rate laws or rate equations are mathematical expressions that describe the relationship between the rate of a chemical reaction and the. The rate therefore has units of. The rate of a reaction is the change in concentration of either the reactant or product with respect to time.
From www.youtube.com
Integrated Rate Laws Zero, First, & Second Order Reactions Chemical What Is Rate Constant In Chemical Kinetics During the course of a reaction, the concentration of the reactants will decrease and. Rate laws or rate equations are mathematical expressions that describe the relationship between the rate of a chemical reaction and the. The rate therefore has units of. The rate constant is a proportionality factor in the rate law that quantifies the speed of a chemical reaction. What Is Rate Constant In Chemical Kinetics.
From www.youtube.com
Intro to Rate Laws, Rate Constants, Reaction Order Chemistry Tutorial What Is Rate Constant In Chemical Kinetics The rate of a reaction is the change in concentration of either the reactant or product with respect to time. The rate therefore has units of. A rate law is an expression showing the relationship of the reaction rate to the concentrations of each reactant. Rate laws or rate equations are mathematical expressions that describe the relationship between the rate. What Is Rate Constant In Chemical Kinetics.
From www.youtube.com
How To Determine The Units Of The Rate Constant K Chemical What Is Rate Constant In Chemical Kinetics The rate constant is a proportionality factor in the rate law that quantifies the speed of a chemical reaction at a given temperature. When we talk about the rate of a chemical reaction, what we mean is the rate at which reactants are used up, or equivalently the rate at which products are formed. During the course of a reaction,. What Is Rate Constant In Chemical Kinetics.
From www.youtube.com
Determine the rate constant (k) for a reaction YouTube What Is Rate Constant In Chemical Kinetics During the course of a reaction, the concentration of the reactants will decrease and. Rate laws or rate equations are mathematical expressions that describe the relationship between the rate of a chemical reaction and the. Rate = k [a] 0. When we talk about the rate of a chemical reaction, what we mean is the rate at which reactants are. What Is Rate Constant In Chemical Kinetics.
From www.youtube.com
Chemical Chapter 14 Part 1 Relative Rate Equations YouTube What Is Rate Constant In Chemical Kinetics During the course of a reaction, the concentration of the reactants will decrease and. The rate constant is a proportionality factor in the rate law that quantifies the speed of a chemical reaction at a given temperature. The rate therefore has units of. When we talk about the rate of a chemical reaction, what we mean is the rate at. What Is Rate Constant In Chemical Kinetics.
From www.youtube.com
Units of the rate constant AP Chemistry Khan Academy What Is Rate Constant In Chemical Kinetics When we talk about the rate of a chemical reaction, what we mean is the rate at which reactants are used up, or equivalently the rate at which products are formed. The rate therefore has units of. The rate of a reaction is the change in concentration of either the reactant or product with respect to time. Rate = k. What Is Rate Constant In Chemical Kinetics.
From www-jmg.ch.cam.ac.uk
notes What Is Rate Constant In Chemical Kinetics The rate of a reaction is the change in concentration of either the reactant or product with respect to time. When we talk about the rate of a chemical reaction, what we mean is the rate at which reactants are used up, or equivalently the rate at which products are formed. Rate = k [a] 0. The rate constant is. What Is Rate Constant In Chemical Kinetics.
From www.slideserve.com
PPT Chemical PowerPoint Presentation, free download ID6810274 What Is Rate Constant In Chemical Kinetics Rate = k [a] 0. The rate constant is a proportionality factor in the rate law that quantifies the speed of a chemical reaction at a given temperature. During the course of a reaction, the concentration of the reactants will decrease and. Rate laws or rate equations are mathematical expressions that describe the relationship between the rate of a chemical. What Is Rate Constant In Chemical Kinetics.
From www.slideserve.com
PPT Chemical PowerPoint Presentation, free download ID5829521 What Is Rate Constant In Chemical Kinetics During the course of a reaction, the concentration of the reactants will decrease and. The rate of a reaction is the change in concentration of either the reactant or product with respect to time. Rate = k [a] 0. A rate law is an expression showing the relationship of the reaction rate to the concentrations of each reactant. The rate. What Is Rate Constant In Chemical Kinetics.
From www.slideserve.com
PPT Chemical PowerPoint Presentation, free download ID315859 What Is Rate Constant In Chemical Kinetics When we talk about the rate of a chemical reaction, what we mean is the rate at which reactants are used up, or equivalently the rate at which products are formed. Rate laws or rate equations are mathematical expressions that describe the relationship between the rate of a chemical reaction and the. The rate therefore has units of. Rate =. What Is Rate Constant In Chemical Kinetics.
From www.slideserve.com
PPT Chapter 15 Chemical The Rates of Chemical Reactions What Is Rate Constant In Chemical Kinetics A rate law is an expression showing the relationship of the reaction rate to the concentrations of each reactant. When we talk about the rate of a chemical reaction, what we mean is the rate at which reactants are used up, or equivalently the rate at which products are formed. During the course of a reaction, the concentration of the. What Is Rate Constant In Chemical Kinetics.
From www.youtube.com
Chemical unit of the rate constant for different order What Is Rate Constant In Chemical Kinetics Rate laws or rate equations are mathematical expressions that describe the relationship between the rate of a chemical reaction and the. The rate therefore has units of. During the course of a reaction, the concentration of the reactants will decrease and. A rate law is an expression showing the relationship of the reaction rate to the concentrations of each reactant.. What Is Rate Constant In Chemical Kinetics.
From schools.aglasem.com
CBSE Notes Class 12 Chemistry Chemical What Is Rate Constant In Chemical Kinetics During the course of a reaction, the concentration of the reactants will decrease and. The rate therefore has units of. The rate constant is a proportionality factor in the rate law that quantifies the speed of a chemical reaction at a given temperature. When we talk about the rate of a chemical reaction, what we mean is the rate at. What Is Rate Constant In Chemical Kinetics.
From www.slideserve.com
PPT CHEMICAL PowerPoint Presentation, free download ID5609537 What Is Rate Constant In Chemical Kinetics When we talk about the rate of a chemical reaction, what we mean is the rate at which reactants are used up, or equivalently the rate at which products are formed. The rate therefore has units of. Rate = k [a] 0. The rate of a reaction is the change in concentration of either the reactant or product with respect. What Is Rate Constant In Chemical Kinetics.
From www.slideserve.com
PPT Chemical PowerPoint Presentation, free download ID2054453 What Is Rate Constant In Chemical Kinetics When we talk about the rate of a chemical reaction, what we mean is the rate at which reactants are used up, or equivalently the rate at which products are formed. A rate law is an expression showing the relationship of the reaction rate to the concentrations of each reactant. The rate therefore has units of. Rate = k [a]. What Is Rate Constant In Chemical Kinetics.
From www.slideserve.com
PPT CHEMICAL PowerPoint Presentation ID5609537 What Is Rate Constant In Chemical Kinetics A rate law is an expression showing the relationship of the reaction rate to the concentrations of each reactant. The rate therefore has units of. Rate laws or rate equations are mathematical expressions that describe the relationship between the rate of a chemical reaction and the. The rate constant is a proportionality factor in the rate law that quantifies the. What Is Rate Constant In Chemical Kinetics.
From www.slideshare.net
Chemical What Is Rate Constant In Chemical Kinetics The rate of a reaction is the change in concentration of either the reactant or product with respect to time. Rate = k [a] 0. The rate therefore has units of. Rate laws or rate equations are mathematical expressions that describe the relationship between the rate of a chemical reaction and the. A rate law is an expression showing the. What Is Rate Constant In Chemical Kinetics.
From www.youtube.com
Chemical units of rate constant YouTube What Is Rate Constant In Chemical Kinetics During the course of a reaction, the concentration of the reactants will decrease and. The rate constant is a proportionality factor in the rate law that quantifies the speed of a chemical reaction at a given temperature. The rate therefore has units of. The rate of a reaction is the change in concentration of either the reactant or product with. What Is Rate Constant In Chemical Kinetics.
From www.bartleby.com
Chemical bartleby What Is Rate Constant In Chemical Kinetics The rate constant is a proportionality factor in the rate law that quantifies the speed of a chemical reaction at a given temperature. Rate = k [a] 0. A rate law is an expression showing the relationship of the reaction rate to the concentrations of each reactant. Rate laws or rate equations are mathematical expressions that describe the relationship between. What Is Rate Constant In Chemical Kinetics.
From www.masterorganicchemistry.com
Chemical Master Organic Chemistry What Is Rate Constant In Chemical Kinetics Rate = k [a] 0. When we talk about the rate of a chemical reaction, what we mean is the rate at which reactants are used up, or equivalently the rate at which products are formed. During the course of a reaction, the concentration of the reactants will decrease and. Rate laws or rate equations are mathematical expressions that describe. What Is Rate Constant In Chemical Kinetics.
From chem.libretexts.org
Chapter 14.4 Using Graphs to Determine Rate Laws, Rate Constants and What Is Rate Constant In Chemical Kinetics The rate therefore has units of. The rate of a reaction is the change in concentration of either the reactant or product with respect to time. A rate law is an expression showing the relationship of the reaction rate to the concentrations of each reactant. During the course of a reaction, the concentration of the reactants will decrease and. When. What Is Rate Constant In Chemical Kinetics.
From www.slideserve.com
PPT Chemical PowerPoint Presentation, free download ID5759826 What Is Rate Constant In Chemical Kinetics The rate therefore has units of. Rate = k [a] 0. A rate law is an expression showing the relationship of the reaction rate to the concentrations of each reactant. During the course of a reaction, the concentration of the reactants will decrease and. The rate constant is a proportionality factor in the rate law that quantifies the speed of. What Is Rate Constant In Chemical Kinetics.
From www.slideserve.com
PPT Chapter 14 Chemical PowerPoint Presentation, free What Is Rate Constant In Chemical Kinetics Rate = k [a] 0. During the course of a reaction, the concentration of the reactants will decrease and. When we talk about the rate of a chemical reaction, what we mean is the rate at which reactants are used up, or equivalently the rate at which products are formed. The rate therefore has units of. The rate of a. What Is Rate Constant In Chemical Kinetics.
From www.slideserve.com
PPT Chemical PowerPoint Presentation, free download ID6305526 What Is Rate Constant In Chemical Kinetics When we talk about the rate of a chemical reaction, what we mean is the rate at which reactants are used up, or equivalently the rate at which products are formed. During the course of a reaction, the concentration of the reactants will decrease and. A rate law is an expression showing the relationship of the reaction rate to the. What Is Rate Constant In Chemical Kinetics.
From www.youtube.com
Writing Rate Laws of Reaction Mechanisms Using The Rate Determining What Is Rate Constant In Chemical Kinetics The rate of a reaction is the change in concentration of either the reactant or product with respect to time. During the course of a reaction, the concentration of the reactants will decrease and. When we talk about the rate of a chemical reaction, what we mean is the rate at which reactants are used up, or equivalently the rate. What Is Rate Constant In Chemical Kinetics.
From www.youtube.com
CHEMICAL (04)!! WHAT IS RATE CONSTANT!! BASIC CONCEPTS YouTube What Is Rate Constant In Chemical Kinetics Rate laws or rate equations are mathematical expressions that describe the relationship between the rate of a chemical reaction and the. The rate constant is a proportionality factor in the rate law that quantifies the speed of a chemical reaction at a given temperature. The rate therefore has units of. Rate = k [a] 0. A rate law is an. What Is Rate Constant In Chemical Kinetics.
From www.youtube.com
CHEMICAL integrated rate equation for zero order reaction What Is Rate Constant In Chemical Kinetics When we talk about the rate of a chemical reaction, what we mean is the rate at which reactants are used up, or equivalently the rate at which products are formed. Rate = k [a] 0. Rate laws or rate equations are mathematical expressions that describe the relationship between the rate of a chemical reaction and the. During the course. What Is Rate Constant In Chemical Kinetics.
From www.slideserve.com
PPT Chapter 16 Rates and Mechanisms of Chemical Reactions What Is Rate Constant In Chemical Kinetics The rate of a reaction is the change in concentration of either the reactant or product with respect to time. The rate therefore has units of. A rate law is an expression showing the relationship of the reaction rate to the concentrations of each reactant. The rate constant is a proportionality factor in the rate law that quantifies the speed. What Is Rate Constant In Chemical Kinetics.
From www.slideserve.com
PPT Chapter 13 Chemical PowerPoint Presentation, free What Is Rate Constant In Chemical Kinetics When we talk about the rate of a chemical reaction, what we mean is the rate at which reactants are used up, or equivalently the rate at which products are formed. Rate laws or rate equations are mathematical expressions that describe the relationship between the rate of a chemical reaction and the. A rate law is an expression showing the. What Is Rate Constant In Chemical Kinetics.
From www.slideserve.com
PPT Topic 15 Chemical PowerPoint Presentation, free What Is Rate Constant In Chemical Kinetics The rate constant is a proportionality factor in the rate law that quantifies the speed of a chemical reaction at a given temperature. During the course of a reaction, the concentration of the reactants will decrease and. Rate laws or rate equations are mathematical expressions that describe the relationship between the rate of a chemical reaction and the. The rate. What Is Rate Constant In Chemical Kinetics.
From www.youtube.com
Rate constant in chemical Rate constant definition define What Is Rate Constant In Chemical Kinetics Rate laws or rate equations are mathematical expressions that describe the relationship between the rate of a chemical reaction and the. The rate therefore has units of. The rate constant is a proportionality factor in the rate law that quantifies the speed of a chemical reaction at a given temperature. During the course of a reaction, the concentration of the. What Is Rate Constant In Chemical Kinetics.
From www.youtube.com
9) Units of Rate constant How to calculate units of Rate constant What Is Rate Constant In Chemical Kinetics The rate of a reaction is the change in concentration of either the reactant or product with respect to time. During the course of a reaction, the concentration of the reactants will decrease and. The rate constant is a proportionality factor in the rate law that quantifies the speed of a chemical reaction at a given temperature. When we talk. What Is Rate Constant In Chemical Kinetics.
From www.youtube.com
AP Chemistry 1 Differential Rate Law & rate constant YouTube What Is Rate Constant In Chemical Kinetics When we talk about the rate of a chemical reaction, what we mean is the rate at which reactants are used up, or equivalently the rate at which products are formed. The rate constant is a proportionality factor in the rate law that quantifies the speed of a chemical reaction at a given temperature. Rate laws or rate equations are. What Is Rate Constant In Chemical Kinetics.
From www.chemistrystudent.com
The Rate Equation (ALevel) ChemistryStudent What Is Rate Constant In Chemical Kinetics Rate laws or rate equations are mathematical expressions that describe the relationship between the rate of a chemical reaction and the. The rate of a reaction is the change in concentration of either the reactant or product with respect to time. The rate therefore has units of. A rate law is an expression showing the relationship of the reaction rate. What Is Rate Constant In Chemical Kinetics.
From www.youtube.com
Chemical Initial Rates Method YouTube What Is Rate Constant In Chemical Kinetics Rate laws or rate equations are mathematical expressions that describe the relationship between the rate of a chemical reaction and the. The rate of a reaction is the change in concentration of either the reactant or product with respect to time. A rate law is an expression showing the relationship of the reaction rate to the concentrations of each reactant.. What Is Rate Constant In Chemical Kinetics.