Baking Soda Solution Chemical Reaction . Carbonic acid and sodium acetate. One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one. The decomposition reaction of sodium bicarbonate or baking soda is an important chemical reaction for baking because it helps baked goods rise. These 2 components react in. When vinegar and baking soda are first mixed together, hydrogen ions in the vinegar react with the sodium and bicarbonate ions in the baking soda. It's also how you can. Baking soda and vinegar react to neutralise each other ( vinegar is an acid and baking soda an alkali ) releasing carbon dioxide which is the bubbles of gas you see. The result of this initial reaction is two new chemicals: These 2 components react in. The second reaction is a decomposition reaction. Balanced chemical equation for baking soda and vinegar reaction. Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid.
from www.chegg.com
It's also how you can. Baking soda and vinegar react to neutralise each other ( vinegar is an acid and baking soda an alkali ) releasing carbon dioxide which is the bubbles of gas you see. The result of this initial reaction is two new chemicals: Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. Carbonic acid and sodium acetate. The decomposition reaction of sodium bicarbonate or baking soda is an important chemical reaction for baking because it helps baked goods rise. The second reaction is a decomposition reaction. Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. Balanced chemical equation for baking soda and vinegar reaction. These 2 components react in.
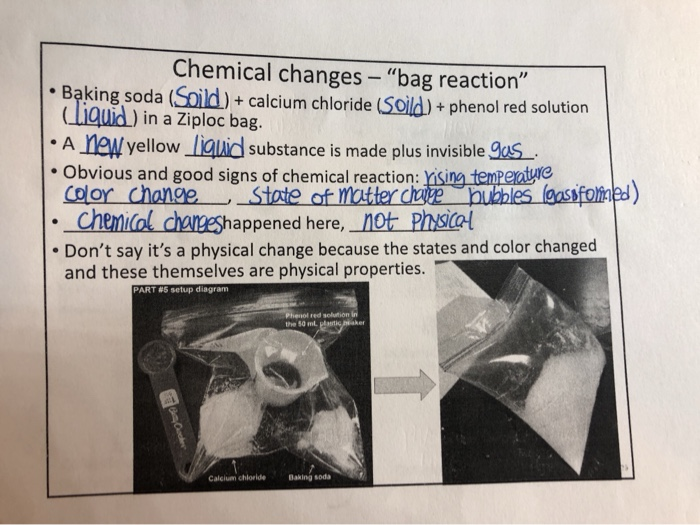
Solved Chemical changes "bag reaction” • Baking soda
Baking Soda Solution Chemical Reaction These 2 components react in. When vinegar and baking soda are first mixed together, hydrogen ions in the vinegar react with the sodium and bicarbonate ions in the baking soda. The result of this initial reaction is two new chemicals: The second reaction is a decomposition reaction. One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one. Carbonic acid and sodium acetate. Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. These 2 components react in. Baking soda and vinegar react to neutralise each other ( vinegar is an acid and baking soda an alkali ) releasing carbon dioxide which is the bubbles of gas you see. The decomposition reaction of sodium bicarbonate or baking soda is an important chemical reaction for baking because it helps baked goods rise. Balanced chemical equation for baking soda and vinegar reaction. These 2 components react in. Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. It's also how you can.
From www.alamy.com
Chemical Reaction of Baking Soda (sodium bicarbonate) and Vinegar Stock Baking Soda Solution Chemical Reaction The second reaction is a decomposition reaction. Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. Balanced chemical equation for baking soda and vinegar reaction. Baking soda and vinegar react to neutralise each other ( vinegar is an acid and baking soda an alkali ) releasing carbon dioxide which is the bubbles of gas. Baking Soda Solution Chemical Reaction.
From www.youtube.com
Chemical reaction mixing baking soda and vinegar science experiment Baking Soda Solution Chemical Reaction The decomposition reaction of sodium bicarbonate or baking soda is an important chemical reaction for baking because it helps baked goods rise. Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. When vinegar and baking soda are first mixed together, hydrogen ions in the vinegar react with the sodium and bicarbonate ions in the. Baking Soda Solution Chemical Reaction.
From www.slideserve.com
PPT Baking Soda and Vinegar Limiting Reactant Lab PowerPoint Baking Soda Solution Chemical Reaction Baking soda and vinegar react to neutralise each other ( vinegar is an acid and baking soda an alkali ) releasing carbon dioxide which is the bubbles of gas you see. Balanced chemical equation for baking soda and vinegar reaction. Carbonic acid and sodium acetate. The decomposition reaction of sodium bicarbonate or baking soda is an important chemical reaction for. Baking Soda Solution Chemical Reaction.
From www.pinterest.com
Vinegar Baking Soda Demonstrations Middle school chemistry, Middle Baking Soda Solution Chemical Reaction These 2 components react in. Carbonic acid and sodium acetate. Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. The result of this initial reaction is two new chemicals: Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. The second reaction is a decomposition reaction. Baking soda and. Baking Soda Solution Chemical Reaction.
From www.youtube.com
Baking Soda and Vinegar Chemical Reaction (EXPERIMENT EXPLAINED) YouTube Baking Soda Solution Chemical Reaction These 2 components react in. These 2 components react in. The decomposition reaction of sodium bicarbonate or baking soda is an important chemical reaction for baking because it helps baked goods rise. Balanced chemical equation for baking soda and vinegar reaction. When vinegar and baking soda are first mixed together, hydrogen ions in the vinegar react with the sodium and. Baking Soda Solution Chemical Reaction.
From printables.it.com
Baking Soda Science Printables Chemical Reaction Free Printable Download Baking Soda Solution Chemical Reaction It's also how you can. Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one. Baking soda and vinegar react to neutralise each other ( vinegar is an acid and baking soda an alkali ) releasing. Baking Soda Solution Chemical Reaction.
From www.numerade.com
SOLVED The chemical equation for the reaction of baking soda (sodium Baking Soda Solution Chemical Reaction Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. These 2 components react in. These 2 components react in. Baking soda and vinegar react to neutralise each other ( vinegar is an acid and baking soda an alkali ) releasing carbon dioxide which is the bubbles of gas you see. Carbonic acid and sodium. Baking Soda Solution Chemical Reaction.
From www.youtube.com
Signs of Chemical Reactions Lab Citric Acid and Baking Soda Temp Change Baking Soda Solution Chemical Reaction These 2 components react in. Balanced chemical equation for baking soda and vinegar reaction. These 2 components react in. The decomposition reaction of sodium bicarbonate or baking soda is an important chemical reaction for baking because it helps baked goods rise. The result of this initial reaction is two new chemicals: It's also how you can. The second reaction is. Baking Soda Solution Chemical Reaction.
From klaxiajzu.blob.core.windows.net
Baking Soda And Vinegar Reaction Chemical Formula at Genevie Barraza blog Baking Soda Solution Chemical Reaction These 2 components react in. These 2 components react in. When vinegar and baking soda are first mixed together, hydrogen ions in the vinegar react with the sodium and bicarbonate ions in the baking soda. Baking soda and vinegar react to neutralise each other ( vinegar is an acid and baking soda an alkali ) releasing carbon dioxide which is. Baking Soda Solution Chemical Reaction.
From www.science-sparks.com
What is the Baking Soda and Vinegar Reaction? Science Sparks Baking Soda Solution Chemical Reaction The second reaction is a decomposition reaction. The result of this initial reaction is two new chemicals: One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one. Baking soda and vinegar react to neutralise each other ( vinegar is an acid and baking soda an alkali ) releasing carbon dioxide which. Baking Soda Solution Chemical Reaction.
From www.pinterest.ca
A Simple Explanation the Difference Between Baking Soda & Baking Baking Soda Solution Chemical Reaction When vinegar and baking soda are first mixed together, hydrogen ions in the vinegar react with the sodium and bicarbonate ions in the baking soda. Carbonic acid and sodium acetate. These 2 components react in. Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. Baking soda and vinegar react to neutralise each other (. Baking Soda Solution Chemical Reaction.
From chemistry291.blogspot.com
Baking Soda and Vinegar Chemical Reaction ExplanationNaHCO3 + CH3COOH Baking Soda Solution Chemical Reaction The result of this initial reaction is two new chemicals: It's also how you can. The second reaction is a decomposition reaction. When vinegar and baking soda are first mixed together, hydrogen ions in the vinegar react with the sodium and bicarbonate ions in the baking soda. One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic. Baking Soda Solution Chemical Reaction.
From chemistry291.blogspot.com
Baking Soda and Vinegar Chemical Reaction ExplanationNaHCO3 + CH3COOH Baking Soda Solution Chemical Reaction When vinegar and baking soda are first mixed together, hydrogen ions in the vinegar react with the sodium and bicarbonate ions in the baking soda. One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one. Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. Baking. Baking Soda Solution Chemical Reaction.
From www.youtube.com
Type of Reaction for Baking Soda and Vinegar ( NaHCO3 + CH3COOH) YouTube Baking Soda Solution Chemical Reaction One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one. The second reaction is a decomposition reaction. It's also how you can. When vinegar and baking soda are first mixed together, hydrogen ions in the vinegar react with the sodium and bicarbonate ions in the baking soda. Baking soda and vinegar. Baking Soda Solution Chemical Reaction.
From www.alamy.com
Chemical reaction of phenolphthalein solution (Baking soda, Vinegar and Baking Soda Solution Chemical Reaction The decomposition reaction of sodium bicarbonate or baking soda is an important chemical reaction for baking because it helps baked goods rise. When vinegar and baking soda are first mixed together, hydrogen ions in the vinegar react with the sodium and bicarbonate ions in the baking soda. The result of this initial reaction is two new chemicals: Balanced chemical equation. Baking Soda Solution Chemical Reaction.
From studylib.net
Reaction of Citric Acid Solution with Baking Soda Baking Soda Solution Chemical Reaction Balanced chemical equation for baking soda and vinegar reaction. Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. The result of this initial reaction is two new chemicals: The decomposition reaction of sodium bicarbonate or baking soda is an important chemical reaction for baking because it helps baked goods rise. The second reaction is. Baking Soda Solution Chemical Reaction.
From www.youtube.com
Citric acid and Baking Soda Reaction YouTube Baking Soda Solution Chemical Reaction One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one. The decomposition reaction of sodium bicarbonate or baking soda is an important chemical reaction for baking because it helps baked goods rise. These 2 components react in. It's also how you can. These 2 components react in. Carbonic acid and sodium. Baking Soda Solution Chemical Reaction.
From www.ingridscience.ca
Baking soda and vinegar ingridscience.ca Baking Soda Solution Chemical Reaction Balanced chemical equation for baking soda and vinegar reaction. Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. It's also how you can. The result of this initial reaction is two new chemicals: One mole of sodium bicarbonate (baking. Baking Soda Solution Chemical Reaction.
From www.youtube.com
Citric acid + baking soda + water reaction YouTube Baking Soda Solution Chemical Reaction One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one. These 2 components react in. The second reaction is a decomposition reaction. Carbonic acid and sodium acetate. Baking soda and vinegar react to neutralise each other ( vinegar is an acid and baking soda an alkali ) releasing carbon dioxide which. Baking Soda Solution Chemical Reaction.
From www.chegg.com
Solved Chemical changes "bag reaction” • Baking soda Baking Soda Solution Chemical Reaction The result of this initial reaction is two new chemicals: These 2 components react in. Carbonic acid and sodium acetate. The second reaction is a decomposition reaction. Balanced chemical equation for baking soda and vinegar reaction. When vinegar and baking soda are first mixed together, hydrogen ions in the vinegar react with the sodium and bicarbonate ions in the baking. Baking Soda Solution Chemical Reaction.
From www.sciencephoto.com
Baking soda reacting with vinegar Stock Image A500/0648 Science Baking Soda Solution Chemical Reaction Balanced chemical equation for baking soda and vinegar reaction. The second reaction is a decomposition reaction. It's also how you can. The decomposition reaction of sodium bicarbonate or baking soda is an important chemical reaction for baking because it helps baked goods rise. When vinegar and baking soda are first mixed together, hydrogen ions in the vinegar react with the. Baking Soda Solution Chemical Reaction.
From www.youtube.com
Chemical reaction mixing baking soda and vinegar YouTube Baking Soda Solution Chemical Reaction Carbonic acid and sodium acetate. When vinegar and baking soda are first mixed together, hydrogen ions in the vinegar react with the sodium and bicarbonate ions in the baking soda. Balanced chemical equation for baking soda and vinegar reaction. Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. The decomposition reaction of sodium bicarbonate. Baking Soda Solution Chemical Reaction.
From www.youtube.com
vinegar and baking soda reaction YouTube Baking Soda Solution Chemical Reaction These 2 components react in. Carbonic acid and sodium acetate. These 2 components react in. Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. It's also how you can. Balanced chemical equation for baking soda and vinegar reaction. When vinegar and baking soda are first mixed together, hydrogen ions in the vinegar react with. Baking Soda Solution Chemical Reaction.
From www.youtube.com
Baking Soda and Vinegar Equation and Reaction YouTube Baking Soda Solution Chemical Reaction These 2 components react in. Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. Baking soda and vinegar react to neutralise each other ( vinegar is an acid and baking soda an alkali ) releasing carbon dioxide which is the bubbles of gas you see. These 2 components react in. One mole of sodium. Baking Soda Solution Chemical Reaction.
From www.alamy.com
Science experiment with baking soda and vinegar balloon illustration Baking Soda Solution Chemical Reaction One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one. The decomposition reaction of sodium bicarbonate or baking soda is an important chemical reaction for baking because it helps baked goods rise. Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. These 2 components react. Baking Soda Solution Chemical Reaction.
From learningmediascouthers.z14.web.core.windows.net
Science Experiment Using Baking Soda Baking Soda Solution Chemical Reaction These 2 components react in. Baking soda and vinegar react to neutralise each other ( vinegar is an acid and baking soda an alkali ) releasing carbon dioxide which is the bubbles of gas you see. Balanced chemical equation for baking soda and vinegar reaction. One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from. Baking Soda Solution Chemical Reaction.
From www.slideserve.com
PPT Baking Soda and Vinegar Limiting Reactant Lab PowerPoint Baking Soda Solution Chemical Reaction Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. Baking soda and vinegar react to neutralise each other ( vinegar is an acid and baking soda an alkali ) releasing carbon dioxide which is the bubbles of gas you see. When vinegar and baking soda are first mixed together, hydrogen ions in the vinegar. Baking Soda Solution Chemical Reaction.
From klazdhlhg.blob.core.windows.net
Baking Soda And Vinegar Reaction Images at Krista Arndt blog Baking Soda Solution Chemical Reaction The result of this initial reaction is two new chemicals: Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. The second reaction is a decomposition reaction. When vinegar and baking soda are first mixed together, hydrogen ions in the vinegar react with the sodium and bicarbonate ions in the baking soda. These 2 components. Baking Soda Solution Chemical Reaction.
From www.youtube.com
Chemical Reaction Of Baking Soda And Vinegar (Sodium Bicarbonate And Baking Soda Solution Chemical Reaction The decomposition reaction of sodium bicarbonate or baking soda is an important chemical reaction for baking because it helps baked goods rise. Baking soda and vinegar react to neutralise each other ( vinegar is an acid and baking soda an alkali ) releasing carbon dioxide which is the bubbles of gas you see. It's also how you can. Carbonic acid. Baking Soda Solution Chemical Reaction.
From www.youtube.com
FLOATING SMOKE Experiment (baking soda & Vinegar chemical reaction Baking Soda Solution Chemical Reaction Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. Baking soda and vinegar react to neutralise each other ( vinegar is an acid and baking soda an alkali ) releasing carbon dioxide which is the bubbles of gas you see. When vinegar and baking soda are first mixed together, hydrogen ions in the vinegar. Baking Soda Solution Chemical Reaction.
From www.funwithmama.com
Baking Soda And Vinegar Reaction Fun with Mama Baking Soda Solution Chemical Reaction Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. It's also how you can. The result of this initial reaction is two new chemicals: These 2 components react in. The second reaction is a decomposition reaction. Baking soda and vinegar react to neutralise each other ( vinegar is an acid and baking soda an. Baking Soda Solution Chemical Reaction.
From sciencenotes.org
Chemical Equation for Baking Soda and Vinegar Reaction Baking Soda Solution Chemical Reaction The decomposition reaction of sodium bicarbonate or baking soda is an important chemical reaction for baking because it helps baked goods rise. The result of this initial reaction is two new chemicals: Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. Baking soda and vinegar react to neutralise each other ( vinegar is an. Baking Soda Solution Chemical Reaction.
From craftsbyamanda.com
Colorful Baking Soda and Vinegar Reaction Crafts by Amanda Easy Baking Soda Solution Chemical Reaction Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. Baking soda and vinegar react to neutralise each other ( vinegar is an acid and baking soda an alkali ) releasing carbon dioxide which is the bubbles of gas you see. The second reaction is a decomposition reaction. Baking soda is a powdered chemical compound. Baking Soda Solution Chemical Reaction.
From chemistry291.blogspot.com
Baking Soda and Vinegar Chemical Reaction ExplanationNaHCO3 + CH3COOH Baking Soda Solution Chemical Reaction Carbonic acid and sodium acetate. Balanced chemical equation for baking soda and vinegar reaction. Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. The result of this initial reaction is two new chemicals: The second reaction is a decomposition reaction. When vinegar and baking soda are first mixed together, hydrogen ions in the vinegar. Baking Soda Solution Chemical Reaction.
From www.thoughtco.com
Turn Baking Soda Into Washing Soda Baking Soda Solution Chemical Reaction It's also how you can. These 2 components react in. Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. The second reaction is a decomposition reaction. One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one. The decomposition reaction of sodium bicarbonate or baking soda. Baking Soda Solution Chemical Reaction.