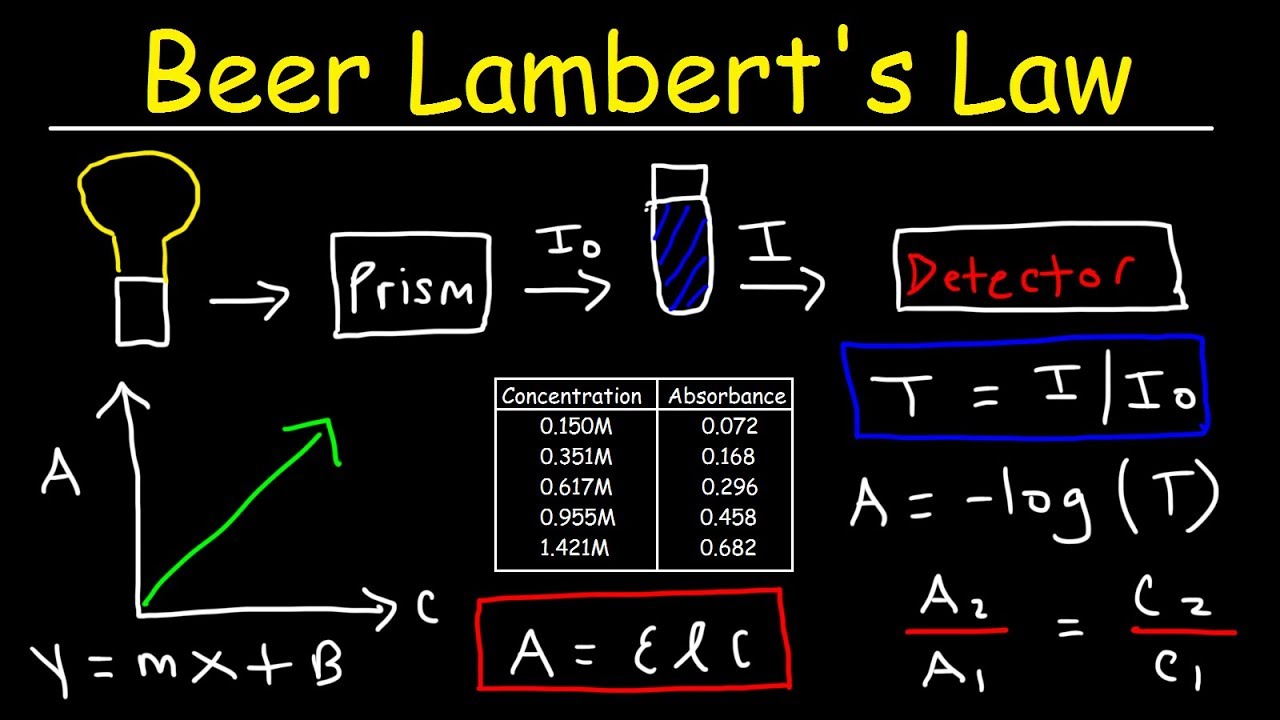
Beer's Law Absorbance . Concentration—we will call this a beer’s law plot—is a straight line with a y. The law states that a chemical's concentration is directly proportional to a solution's absorbance. You may use this relation to determine a chemical species' concentration in a solution using a colorimeter or spectrophotometer. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. The absorbance is related to the ratio of the intensity of light that enters the sample and leaves the sample. Beer's law is an equation that relates light's attenuation to a material's properties. Beer’s law suggests that a plot of absorbance vs.
from www.youtube.com
Beer's law is an equation that relates light's attenuation to a material's properties. You may use this relation to determine a chemical species' concentration in a solution using a colorimeter or spectrophotometer. Concentration—we will call this a beer’s law plot—is a straight line with a y. The law states that a chemical's concentration is directly proportional to a solution's absorbance. The absorbance is related to the ratio of the intensity of light that enters the sample and leaves the sample. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer’s law suggests that a plot of absorbance vs.
Beer Lambert's Law, Absorbance & Transmittance Spectrophotometry
Beer's Law Absorbance Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. The law states that a chemical's concentration is directly proportional to a solution's absorbance. Beer’s law suggests that a plot of absorbance vs. Beer's law is an equation that relates light's attenuation to a material's properties. You may use this relation to determine a chemical species' concentration in a solution using a colorimeter or spectrophotometer. Concentration—we will call this a beer’s law plot—is a straight line with a y. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. The absorbance is related to the ratio of the intensity of light that enters the sample and leaves the sample.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download Beer's Law Absorbance Concentration—we will call this a beer’s law plot—is a straight line with a y. You may use this relation to determine a chemical species' concentration in a solution using a colorimeter or spectrophotometer. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer's law is an equation that relates. Beer's Law Absorbance.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download Beer's Law Absorbance Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. The absorbance is related to the ratio of the intensity of light that enters the sample and leaves the sample. The law states that a chemical's concentration is directly proportional to a solution's absorbance. Concentration—we will call this a beer’s. Beer's Law Absorbance.
From www.youtube.com
Beer Lambert's Law, Absorbance & Transmittance Spectrophotometry Beer's Law Absorbance You may use this relation to determine a chemical species' concentration in a solution using a colorimeter or spectrophotometer. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. The absorbance is related to the ratio of the intensity of light that enters the sample and leaves the sample. Concentration—we. Beer's Law Absorbance.
From www.researchgate.net
Measuring transmittance and absorbance using BeerLambert's law Beer's Law Absorbance Concentration—we will call this a beer’s law plot—is a straight line with a y. Beer's law is an equation that relates light's attenuation to a material's properties. You may use this relation to determine a chemical species' concentration in a solution using a colorimeter or spectrophotometer. The absorbance is related to the ratio of the intensity of light that enters. Beer's Law Absorbance.
From www.researchgate.net
(a) Calculated absorbance unit by BeerLambert Law for A. hydrophila Beer's Law Absorbance Concentration—we will call this a beer’s law plot—is a straight line with a y. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. The absorbance is related to the ratio of the intensity of light that enters the sample and leaves the sample. The law states that a chemical's. Beer's Law Absorbance.
From sciencenotes.org
Beer's Law Equation and Example Beer's Law Absorbance Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. You may use this relation to determine a chemical species' concentration in a solution using a colorimeter or spectrophotometer. The absorbance is related to the ratio of the intensity of light that enters the sample and leaves the sample. Beer's. Beer's Law Absorbance.
From slidetodoc.com
Part 2 9 Electronic Transitions Outline Absorption spectroscopy Beer's Law Absorbance Beer’s law suggests that a plot of absorbance vs. Beer's law is an equation that relates light's attenuation to a material's properties. The law states that a chemical's concentration is directly proportional to a solution's absorbance. The absorbance is related to the ratio of the intensity of light that enters the sample and leaves the sample. You may use this. Beer's Law Absorbance.
From cevhwrun.blob.core.windows.net
Beer Lambert Law Relationship Between Absorbance And Concentration at Beer's Law Absorbance Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Concentration—we will call this a beer’s law plot—is a straight line with a y. The absorbance is related to the ratio of the intensity of light that enters the sample and leaves the sample. Beer’s law suggests that a plot. Beer's Law Absorbance.
From chemistrytalk.org
BeerLambert Law ChemTalk Beer's Law Absorbance Concentration—we will call this a beer’s law plot—is a straight line with a y. The law states that a chemical's concentration is directly proportional to a solution's absorbance. Beer’s law suggests that a plot of absorbance vs. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer's law is. Beer's Law Absorbance.
From lukewarmtakes.net
Spectrophotometry and Beer's Law Lukewarm Takes Beer's Law Absorbance Beer’s law suggests that a plot of absorbance vs. Beer's law is an equation that relates light's attenuation to a material's properties. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Concentration—we will call this a beer’s law plot—is a straight line with a y. The law states that. Beer's Law Absorbance.
From mavink.com
Absorbance Vs. Concentration Graph Beer's Law Absorbance Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer's law is an equation that relates light's attenuation to a material's properties. Concentration—we will call this a beer’s law plot—is a straight line with a y. The absorbance is related to the ratio of the intensity of light that. Beer's Law Absorbance.
From cevhwrun.blob.core.windows.net
Beer Lambert Law Relationship Between Absorbance And Concentration at Beer's Law Absorbance Concentration—we will call this a beer’s law plot—is a straight line with a y. Beer's law is an equation that relates light's attenuation to a material's properties. The law states that a chemical's concentration is directly proportional to a solution's absorbance. The absorbance is related to the ratio of the intensity of light that enters the sample and leaves the. Beer's Law Absorbance.
From www.edinst.com
Beer Lambert Law Transmittance & Absorbance Edinburgh Instruments Beer's Law Absorbance Beer’s law suggests that a plot of absorbance vs. The law states that a chemical's concentration is directly proportional to a solution's absorbance. You may use this relation to determine a chemical species' concentration in a solution using a colorimeter or spectrophotometer. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write. Beer's Law Absorbance.
From webapi.bu.edu
💄 Beer lambert law absorption. Beer’s Law. 20221109 Beer's Law Absorbance The absorbance is related to the ratio of the intensity of light that enters the sample and leaves the sample. Beer’s law suggests that a plot of absorbance vs. Concentration—we will call this a beer’s law plot—is a straight line with a y. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can. Beer's Law Absorbance.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download Beer's Law Absorbance Beer’s law suggests that a plot of absorbance vs. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. The law states that a chemical's concentration is directly proportional to a solution's absorbance. The absorbance is related to the ratio of the intensity of light that enters the sample and. Beer's Law Absorbance.
From www.youtube.com
Beer Law Lambert Law Limiting Law Absorbance Transmittance Beer's Law Absorbance Beer’s law suggests that a plot of absorbance vs. The absorbance is related to the ratio of the intensity of light that enters the sample and leaves the sample. Concentration—we will call this a beer’s law plot—is a straight line with a y. The law states that a chemical's concentration is directly proportional to a solution's absorbance. Since the concentration,. Beer's Law Absorbance.
From quizinterworks.z21.web.core.windows.net
How To Determine Absorbance Beer's Law Absorbance Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. The law states that a chemical's concentration is directly proportional to a solution's absorbance. Concentration—we will call this a beer’s law plot—is a straight line with a y. You may use this relation to determine a chemical species' concentration in. Beer's Law Absorbance.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download Beer's Law Absorbance Concentration—we will call this a beer’s law plot—is a straight line with a y. The law states that a chemical's concentration is directly proportional to a solution's absorbance. Beer's law is an equation that relates light's attenuation to a material's properties. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the. Beer's Law Absorbance.
From www.researchgate.net
1 a Schematic representation for BeerLambert law for the measurement Beer's Law Absorbance You may use this relation to determine a chemical species' concentration in a solution using a colorimeter or spectrophotometer. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. The absorbance is related to the ratio of the intensity of light that enters the sample and leaves the sample. Concentration—we. Beer's Law Absorbance.
From study.com
How to Find the Absorbance of a Solution Using the BeerLambert Law Beer's Law Absorbance Beer’s law suggests that a plot of absorbance vs. Concentration—we will call this a beer’s law plot—is a straight line with a y. The absorbance is related to the ratio of the intensity of light that enters the sample and leaves the sample. The law states that a chemical's concentration is directly proportional to a solution's absorbance. Since the concentration,. Beer's Law Absorbance.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download Beer's Law Absorbance Beer's law is an equation that relates light's attenuation to a material's properties. Concentration—we will call this a beer’s law plot—is a straight line with a y. Beer’s law suggests that a plot of absorbance vs. You may use this relation to determine a chemical species' concentration in a solution using a colorimeter or spectrophotometer. The absorbance is related to. Beer's Law Absorbance.
From www.youtube.com
Beer's Law Absorbance vs Concentration YouTube Beer's Law Absorbance Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. The law states that a chemical's concentration is directly proportional to a solution's absorbance. Concentration—we will call this a beer’s law plot—is a straight line with a y. You may use this relation to determine a chemical species' concentration in. Beer's Law Absorbance.
From www.youtube.com
Absorbance Transmittance Numerical Practice problem on Lambert Beer Beer's Law Absorbance The law states that a chemical's concentration is directly proportional to a solution's absorbance. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Concentration—we will call this a beer’s law plot—is a straight line with a y. Beer’s law suggests that a plot of absorbance vs. Beer's law is. Beer's Law Absorbance.
From www.youtube.com
Absorption law (ii) Beer’s Law UV and Visible Spectroscopy YouTube Beer's Law Absorbance The absorbance is related to the ratio of the intensity of light that enters the sample and leaves the sample. The law states that a chemical's concentration is directly proportional to a solution's absorbance. Beer's law is an equation that relates light's attenuation to a material's properties. Since the concentration, path length and molar absorptivity are all directly proportional to. Beer's Law Absorbance.
From www.thoughtco.com
Beer's Law Definition and Equation Beer's Law Absorbance You may use this relation to determine a chemical species' concentration in a solution using a colorimeter or spectrophotometer. The law states that a chemical's concentration is directly proportional to a solution's absorbance. Beer's law is an equation that relates light's attenuation to a material's properties. Beer’s law suggests that a plot of absorbance vs. Concentration—we will call this a. Beer's Law Absorbance.
From www.edinst.com
Beer Lambert Law Transmittance & Absorbance Edinburgh Instruments Beer's Law Absorbance The absorbance is related to the ratio of the intensity of light that enters the sample and leaves the sample. Beer's law is an equation that relates light's attenuation to a material's properties. The law states that a chemical's concentration is directly proportional to a solution's absorbance. You may use this relation to determine a chemical species' concentration in a. Beer's Law Absorbance.
From www.adda247.com
Beer Lambert Law Equation Derivation, Formula, Examples Beer's Law Absorbance The absorbance is related to the ratio of the intensity of light that enters the sample and leaves the sample. Beer's law is an equation that relates light's attenuation to a material's properties. Beer’s law suggests that a plot of absorbance vs. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write. Beer's Law Absorbance.
From www.edinst.com
Beer Lambert Law Transmittance & Absorbance Edinburgh Instruments Beer's Law Absorbance The law states that a chemical's concentration is directly proportional to a solution's absorbance. You may use this relation to determine a chemical species' concentration in a solution using a colorimeter or spectrophotometer. The absorbance is related to the ratio of the intensity of light that enters the sample and leaves the sample. Beer’s law suggests that a plot of. Beer's Law Absorbance.
From www.thoughtco.com
Beer's Law Definition and Equation Beer's Law Absorbance The absorbance is related to the ratio of the intensity of light that enters the sample and leaves the sample. Beer's law is an equation that relates light's attenuation to a material's properties. The law states that a chemical's concentration is directly proportional to a solution's absorbance. Beer’s law suggests that a plot of absorbance vs. Since the concentration, path. Beer's Law Absorbance.
From cevhwrun.blob.core.windows.net
Beer Lambert Law Relationship Between Absorbance And Concentration at Beer's Law Absorbance The law states that a chemical's concentration is directly proportional to a solution's absorbance. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. You may use this relation to determine a chemical species' concentration in a solution using a colorimeter or spectrophotometer. Beer’s law suggests that a plot of. Beer's Law Absorbance.
From cevhwrun.blob.core.windows.net
Beer Lambert Law Relationship Between Absorbance And Concentration at Beer's Law Absorbance Beer’s law suggests that a plot of absorbance vs. The law states that a chemical's concentration is directly proportional to a solution's absorbance. Concentration—we will call this a beer’s law plot—is a straight line with a y. The absorbance is related to the ratio of the intensity of light that enters the sample and leaves the sample. You may use. Beer's Law Absorbance.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download Beer's Law Absorbance The law states that a chemical's concentration is directly proportional to a solution's absorbance. The absorbance is related to the ratio of the intensity of light that enters the sample and leaves the sample. Beer’s law suggests that a plot of absorbance vs. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can. Beer's Law Absorbance.
From chem.libretexts.org
8.2 Beer's Law Chemistry LibreTexts Beer's Law Absorbance The law states that a chemical's concentration is directly proportional to a solution's absorbance. Concentration—we will call this a beer’s law plot—is a straight line with a y. Beer's law is an equation that relates light's attenuation to a material's properties. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the. Beer's Law Absorbance.
From www.youtube.com
Beer's Law Overview YouTube Beer's Law Absorbance Beer’s law suggests that a plot of absorbance vs. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer's law is an equation that relates light's attenuation to a material's properties. The absorbance is related to the ratio of the intensity of light that enters the sample and leaves. Beer's Law Absorbance.
From www.edinst.com
Beer Lambert Law Transmittance & Absorbance Edinburgh Instruments Beer's Law Absorbance You may use this relation to determine a chemical species' concentration in a solution using a colorimeter or spectrophotometer. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer's law is an equation that relates light's attenuation to a material's properties. Concentration—we will call this a beer’s law plot—is. Beer's Law Absorbance.