Dilution Meaning Class 9 . Dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. Concentration is the removal of solvent, which increases the. It involves the process of decreasing the. Dilution refers to a drop in the ph of a chemical which can be a gas, vapour or solution. You can add water to concentrated orange juice to. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. The dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a solvent to achieve a desired. In this chemistry article, learn about the dilution definition, the serial dilution method, dilution formulas, the importance of dilution, and the enthalpy of dilution. Dilution is the process of reducing the concentration of a given solute in its solution. Dilution of solutions | ch#6 | 9th class chemistry#solution #9thclasschemistry. The chemist can do it simply by mixing with more.
from www.doubtnut.com
Concentration is the removal of solvent, which increases the. Dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. In this chemistry article, learn about the dilution definition, the serial dilution method, dilution formulas, the importance of dilution, and the enthalpy of dilution. The chemist can do it simply by mixing with more. You can add water to concentrated orange juice to. It involves the process of decreasing the. The dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a solvent to achieve a desired. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the process of reducing the concentration of a given solute in its solution. Dilution of solutions | ch#6 | 9th class chemistry#solution #9thclasschemistry.
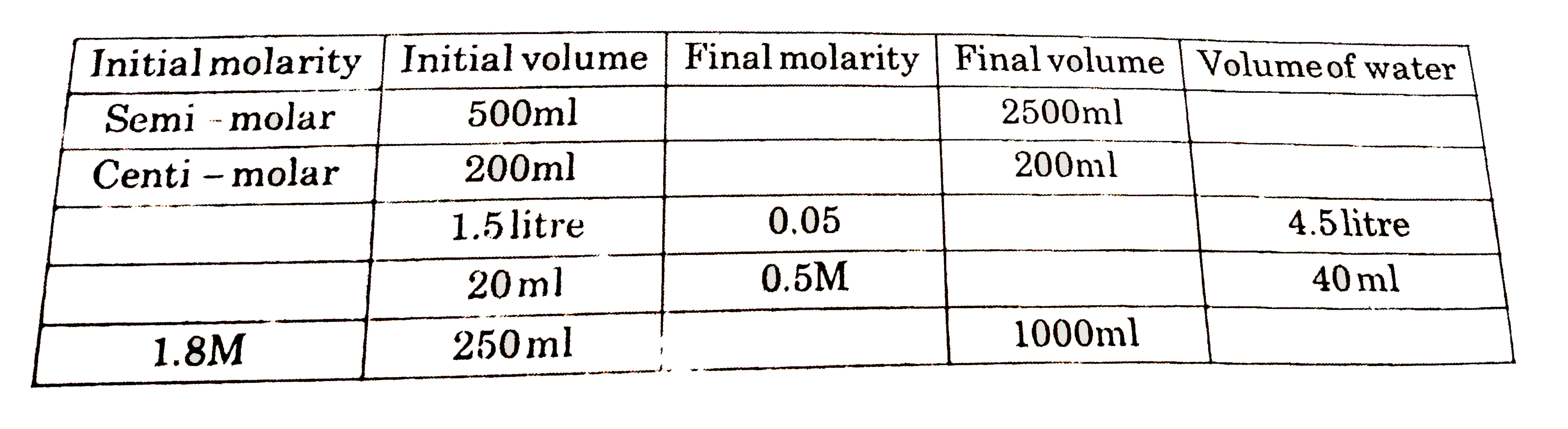
Use of dilution formula (M(1)V(1) = M(2) V(2))
Dilution Meaning Class 9 Dilution of solutions | ch#6 | 9th class chemistry#solution #9thclasschemistry. Dilution of solutions | ch#6 | 9th class chemistry#solution #9thclasschemistry. Dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. Dilution is the process of reducing the concentration of a given solute in its solution. Dilution refers to a drop in the ph of a chemical which can be a gas, vapour or solution. It involves the process of decreasing the. You can add water to concentrated orange juice to. In this chemistry article, learn about the dilution definition, the serial dilution method, dilution formulas, the importance of dilution, and the enthalpy of dilution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. The chemist can do it simply by mixing with more. The dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a solvent to achieve a desired. Concentration is the removal of solvent, which increases the.
From www.doubtnut.com
Use of dilution formula (M(1)V(1) = M(2) V(2)) Dilution Meaning Class 9 You can add water to concentrated orange juice to. In this chemistry article, learn about the dilution definition, the serial dilution method, dilution formulas, the importance of dilution, and the enthalpy of dilution. Dilution refers to a drop in the ph of a chemical which can be a gas, vapour or solution. Concentration is the removal of solvent, which increases. Dilution Meaning Class 9.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory Dilution Meaning Class 9 In this chemistry article, learn about the dilution definition, the serial dilution method, dilution formulas, the importance of dilution, and the enthalpy of dilution. It involves the process of decreasing the. Dilution is the process of reducing the concentration of a given solute in its solution. Concentration is the removal of solvent, which increases the. Dilution is the addition of. Dilution Meaning Class 9.
From www.slideserve.com
PPT Study Guide for Dilution PROBLEMS and Concentrations problems Dilution Meaning Class 9 Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. Dilution refers to a drop in the ph of a chemical which can be a gas, vapour or solution. Concentration is the removal of. Dilution Meaning Class 9.
From www.slideserve.com
PPT Dilution and Evaporation PowerPoint Presentation, free download Dilution Meaning Class 9 It involves the process of decreasing the. The dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a solvent to achieve a desired. In this chemistry article, learn about the dilution definition, the serial dilution method, dilution formulas, the importance of dilution, and the enthalpy of dilution. Dilution is the process of reducing the concentration. Dilution Meaning Class 9.
From ar.inspiredpencil.com
Dilute Science Dilution Meaning Class 9 Dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. Dilution refers to a drop in the ph of a chemical which can be a gas, vapour or solution. Dilution is the process of reducing the concentration of a given solute in its solution. It involves the process of decreasing. Dilution Meaning Class 9.
From www.youtube.com
Unit 8.7 Dilution and Examples YouTube Dilution Meaning Class 9 The dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a solvent to achieve a desired. Dilution refers to a drop in the ph of a chemical which can be a gas, vapour or solution. Dilution of solutions | ch#6 | 9th class chemistry#solution #9thclasschemistry. Dilution is the addition of solvent, which decreases the concentration. Dilution Meaning Class 9.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube Dilution Meaning Class 9 Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. It involves the process of decreasing the. Dilution is the process of reducing the concentration of a given solute in its solution. In this chemistry article, learn about the dilution definition, the serial dilution method, dilution formulas, the importance of dilution, and the enthalpy. Dilution Meaning Class 9.
From www.sliderbase.com
Strengths of Acids and Bases Making Dilutions Presentation Chemistry Dilution Meaning Class 9 Dilution refers to a drop in the ph of a chemical which can be a gas, vapour or solution. Concentration is the removal of solvent, which increases the. Dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. Dilution of solutions | ch#6 | 9th class chemistry#solution #9thclasschemistry. It involves. Dilution Meaning Class 9.
From www.majordifferences.com
Difference between Dilution and Dilution Factor in Microbiology Dilution Meaning Class 9 It involves the process of decreasing the. You can add water to concentrated orange juice to. The dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a solvent to achieve a desired. Dilution refers to a drop in the ph of a chemical which can be a gas, vapour or solution. Concentration is the removal. Dilution Meaning Class 9.
From www.youtube.com
Dilutions YouTube Dilution Meaning Class 9 The chemist can do it simply by mixing with more. It involves the process of decreasing the. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. The dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a solvent to achieve a desired. Dilution of solutions | ch#6 |. Dilution Meaning Class 9.
From www.teachoo.com
Classification of Acids on Basis of source, Concentration Teachoo Dilution Meaning Class 9 It involves the process of decreasing the. You can add water to concentrated orange juice to. The dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a solvent to achieve a desired. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution refers to a drop in the. Dilution Meaning Class 9.
From www.slideserve.com
PPT Study Guide for Dilution PROBLEMS and Concentrations problems Dilution Meaning Class 9 You can add water to concentrated orange juice to. Concentration is the removal of solvent, which increases the. In this chemistry article, learn about the dilution definition, the serial dilution method, dilution formulas, the importance of dilution, and the enthalpy of dilution. Dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with. Dilution Meaning Class 9.
From www.youtube.com
Dilution and Dilution Factor in Microbiology How to Calculate Dilution Meaning Class 9 Dilution is the process of reducing the concentration of a given solute in its solution. Dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. Dilution refers to a drop in the ph of a chemical which can be a gas, vapour or solution. The chemist can do it simply. Dilution Meaning Class 9.
From www.medicine.mcgill.ca
Serial Dilutions Dilution Meaning Class 9 Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution refers to a drop in the ph of a chemical which can be a gas, vapour or solution. Dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. Concentration is the removal of. Dilution Meaning Class 9.
From exopkbipv.blob.core.windows.net
Dilution Equation Example Chemistry at Wanda King blog Dilution Meaning Class 9 The chemist can do it simply by mixing with more. It involves the process of decreasing the. The dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a solvent to achieve a desired. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. In this chemistry article, learn about. Dilution Meaning Class 9.
From www.chegg.com
Solved Dilution diagrams can be very helpful in organizing Dilution Meaning Class 9 Concentration is the removal of solvent, which increases the. Dilution is the process of reducing the concentration of a given solute in its solution. Dilution refers to a drop in the ph of a chemical which can be a gas, vapour or solution. Dilution of solutions | ch#6 | 9th class chemistry#solution #9thclasschemistry. You can add water to concentrated orange. Dilution Meaning Class 9.
From www.ck12.org
Dilution (M[i]V[i]=M[f]V[f]) Overview ( Video ) Chemistry CK12 Dilution Meaning Class 9 Dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. In this chemistry article, learn about the dilution definition, the serial dilution method, dilution formulas, the importance of dilution, and the enthalpy of dilution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution.. Dilution Meaning Class 9.
From www.youtube.com
What Is Dilution? Chemistry Matters YouTube Dilution Meaning Class 9 Concentration is the removal of solvent, which increases the. In this chemistry article, learn about the dilution definition, the serial dilution method, dilution formulas, the importance of dilution, and the enthalpy of dilution. It involves the process of decreasing the. Dilution is the process of reducing the concentration of a given solute in its solution. The dilution formula (c1v1 =. Dilution Meaning Class 9.
From www.slideserve.com
PPT Study Guide for Dilution PROBLEMS and Concentrations problems Dilution Meaning Class 9 Dilution refers to a drop in the ph of a chemical which can be a gas, vapour or solution. You can add water to concentrated orange juice to. It involves the process of decreasing the. The chemist can do it simply by mixing with more. In this chemistry article, learn about the dilution definition, the serial dilution method, dilution formulas,. Dilution Meaning Class 9.
From mungfali.com
10 Fold Serial Dilution Dilution Meaning Class 9 Dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. In this chemistry article, learn about the dilution definition, the serial dilution method, dilution formulas, the importance of dilution, and the enthalpy of dilution. You can add water to concentrated orange juice to. Dilution is the process of reducing the. Dilution Meaning Class 9.
From carlosgokeowen.blogspot.com
What is Dilution Dilution Meaning Class 9 Concentration is the removal of solvent, which increases the. In this chemistry article, learn about the dilution definition, the serial dilution method, dilution formulas, the importance of dilution, and the enthalpy of dilution. Dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. The dilution formula (c1v1 = c2v2) calculates. Dilution Meaning Class 9.
From www.askiitians.com
Types Of Solutions Study Material for IIT JEE askIITians Dilution Meaning Class 9 Dilution refers to a drop in the ph of a chemical which can be a gas, vapour or solution. The chemist can do it simply by mixing with more. Concentration is the removal of solvent, which increases the. It involves the process of decreasing the. In this chemistry article, learn about the dilution definition, the serial dilution method, dilution formulas,. Dilution Meaning Class 9.
From dxogrtxrg.blob.core.windows.net
Dilution Example In Chemistry at Ignacio Alvarez blog Dilution Meaning Class 9 Dilution is the process of reducing the concentration of a given solute in its solution. The dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a solvent to achieve a desired. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which increases. Dilution Meaning Class 9.
From www.slideserve.com
PPT Dilution PowerPoint Presentation, free download ID6016027 Dilution Meaning Class 9 The dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a solvent to achieve a desired. Concentration is the removal of solvent, which increases the. Dilution is the process of reducing the concentration of a given solute in its solution. Dilution is the addition of solvent, which decreases the concentration of the solute in the. Dilution Meaning Class 9.
From www.youtube.com
DILUTION Meaning and Pronunciation YouTube Dilution Meaning Class 9 The chemist can do it simply by mixing with more. In this chemistry article, learn about the dilution definition, the serial dilution method, dilution formulas, the importance of dilution, and the enthalpy of dilution. Dilution of solutions | ch#6 | 9th class chemistry#solution #9thclasschemistry. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution.. Dilution Meaning Class 9.
From zhtutorials.com
Serial Dilution Practical Skills Ep 3 Zoë Huggett Tutorials Dilution Meaning Class 9 The dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a solvent to achieve a desired. You can add water to concentrated orange juice to. Dilution of solutions | ch#6 | 9th class chemistry#solution #9thclasschemistry. In this chemistry article, learn about the dilution definition, the serial dilution method, dilution formulas, the importance of dilution, and. Dilution Meaning Class 9.
From study.com
Dilution Definition, Equation & Factors Video & Lesson Transcript Dilution Meaning Class 9 Dilution of solutions | ch#6 | 9th class chemistry#solution #9thclasschemistry. Dilution is the process of reducing the concentration of a given solute in its solution. You can add water to concentrated orange juice to. The dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a solvent to achieve a desired. It involves the process of. Dilution Meaning Class 9.
From www.pinterest.com
Dilution when solvent is added to dilute a solution, the number of Dilution Meaning Class 9 The chemist can do it simply by mixing with more. Dilution of solutions | ch#6 | 9th class chemistry#solution #9thclasschemistry. Concentration is the removal of solvent, which increases the. It involves the process of decreasing the. In this chemistry article, learn about the dilution definition, the serial dilution method, dilution formulas, the importance of dilution, and the enthalpy of dilution.. Dilution Meaning Class 9.
From www.youtube.com
Dilution Chart.Helpful video. Understand how to prepare dilutions in Dilution Meaning Class 9 Dilution of solutions | ch#6 | 9th class chemistry#solution #9thclasschemistry. Concentration is the removal of solvent, which increases the. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. It involves the process of decreasing the. You can add water to concentrated orange juice to. The chemist can do it simply by mixing with. Dilution Meaning Class 9.
From joijqqtnu.blob.core.windows.net
Dilution Land Meaning at Bernice Blalock blog Dilution Meaning Class 9 The chemist can do it simply by mixing with more. You can add water to concentrated orange juice to. Concentration is the removal of solvent, which increases the. The dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a solvent to achieve a desired. Dilution of solutions | ch#6 | 9th class chemistry#solution #9thclasschemistry. In. Dilution Meaning Class 9.
From dxodouqth.blob.core.windows.net
Dilution Of Control In English at Richard Blackford blog Dilution Meaning Class 9 You can add water to concentrated orange juice to. The dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a solvent to achieve a desired. The chemist can do it simply by mixing with more. Dilution is the process of reducing the concentration of a given solute in its solution. Dilution refers to a drop. Dilution Meaning Class 9.
From www.toppr.com
Dilution Formula Definition, Concepts and Examples Dilution Meaning Class 9 Concentration is the removal of solvent, which increases the. Dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. Dilution is the process of reducing the concentration of a given solute in its solution. In this chemistry article, learn about the dilution definition, the serial dilution method, dilution formulas, the. Dilution Meaning Class 9.
From www.w3schools.blog
Standard enthalpy of dilution W3schools Dilution Meaning Class 9 Dilution refers to a drop in the ph of a chemical which can be a gas, vapour or solution. Concentration is the removal of solvent, which increases the. Dilution is the process of reducing the concentration of a given solute in its solution. The chemist can do it simply by mixing with more. It involves the process of decreasing the.. Dilution Meaning Class 9.
From www.slashdotblog.com
What Is Dilution? Dilution Meaning Class 9 Dilution of solutions | ch#6 | 9th class chemistry#solution #9thclasschemistry. You can add water to concentrated orange juice to. The dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a solvent to achieve a desired. Dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent.. Dilution Meaning Class 9.
From www.slideserve.com
PPT Dilutions PowerPoint Presentation ID226520 Dilution Meaning Class 9 Concentration is the removal of solvent, which increases the. Dilution refers to a drop in the ph of a chemical which can be a gas, vapour or solution. Dilution of solutions | ch#6 | 9th class chemistry#solution #9thclasschemistry. Dilution is the process of reducing the concentration of a given solute in its solution. Dilution is the addition of solvent, which. Dilution Meaning Class 9.