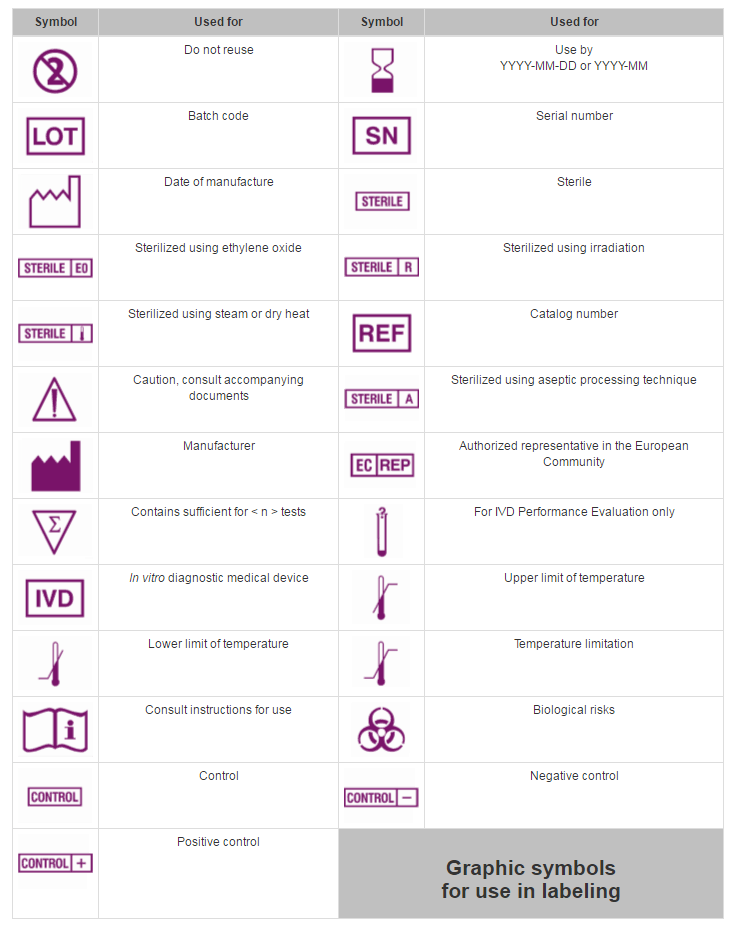
Medical Device Labeling Requirements Font Size . Overall, the agency recommends that the font size should be no smaller than 10 points for any section of the ifu, except no. (a) the label of a device in package. Name and place of business of manufacturer, packer or distributor. In the us and the eu, the requirements for medical device labeling are detailed and extensive, and may be specific. What are the requirements for medical device labeling? Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to.
from exoptcoyn.blob.core.windows.net
What are the requirements for medical device labeling? Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. Name and place of business of manufacturer, packer or distributor. (a) the label of a device in package. In the us and the eu, the requirements for medical device labeling are detailed and extensive, and may be specific. Overall, the agency recommends that the font size should be no smaller than 10 points for any section of the ifu, except no.
Medical Device Label Font Size at Loren Pierce blog
Medical Device Labeling Requirements Font Size (a) the label of a device in package. Name and place of business of manufacturer, packer or distributor. (a) the label of a device in package. In the us and the eu, the requirements for medical device labeling are detailed and extensive, and may be specific. What are the requirements for medical device labeling? Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. Overall, the agency recommends that the font size should be no smaller than 10 points for any section of the ifu, except no.
From www.greenlight.guru
FDA Medical Device Labeling Requirements An Overview Medical Device Labeling Requirements Font Size Overall, the agency recommends that the font size should be no smaller than 10 points for any section of the ifu, except no. (a) the label of a device in package. In the us and the eu, the requirements for medical device labeling are detailed and extensive, and may be specific. What are the requirements for medical device labeling? Name. Medical Device Labeling Requirements Font Size.
From exogmsrpv.blob.core.windows.net
Medical Device Labeling Font Size at Scott Bunger blog Medical Device Labeling Requirements Font Size Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. In the us and the eu, the requirements for medical device labeling are detailed and extensive, and may be specific. What are the requirements for medical device labeling? (a) the label of a device in package. Name and place of business. Medical Device Labeling Requirements Font Size.
From mavink.com
Medical Device Labeling Symbols Medical Device Labeling Requirements Font Size In the us and the eu, the requirements for medical device labeling are detailed and extensive, and may be specific. What are the requirements for medical device labeling? Overall, the agency recommends that the font size should be no smaller than 10 points for any section of the ifu, except no. (a) the label of a device in package. Name. Medical Device Labeling Requirements Font Size.
From www.techsollifesciences.com
EU MDR & IVDR Medical Device Labelling Requirements Medical Device Labeling Requirements Font Size Name and place of business of manufacturer, packer or distributor. In the us and the eu, the requirements for medical device labeling are detailed and extensive, and may be specific. What are the requirements for medical device labeling? (a) the label of a device in package. Elements of medical device and ivd medical device labelling ras require and specify information. Medical Device Labeling Requirements Font Size.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Medical Device Labeling Requirements Font Size Overall, the agency recommends that the font size should be no smaller than 10 points for any section of the ifu, except no. (a) the label of a device in package. In the us and the eu, the requirements for medical device labeling are detailed and extensive, and may be specific. Elements of medical device and ivd medical device labelling. Medical Device Labeling Requirements Font Size.
From asiaactual.com
Hong Kong Medical Device Labeling Requirements Asia Actual, LLC Medical Device Labeling Requirements Font Size Name and place of business of manufacturer, packer or distributor. In the us and the eu, the requirements for medical device labeling are detailed and extensive, and may be specific. Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. What are the requirements for medical device labeling? (a) the label. Medical Device Labeling Requirements Font Size.
From www.regdesk.co
FDA on General Principles of Labeling for Medical Devices RegDesk Medical Device Labeling Requirements Font Size Name and place of business of manufacturer, packer or distributor. What are the requirements for medical device labeling? Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. Overall, the agency recommends that the font size should be no smaller than 10 points for any section of the ifu, except no.. Medical Device Labeling Requirements Font Size.
From www.greenlight.guru
FDA Medical Device Labeling Requirements An Overview Medical Device Labeling Requirements Font Size In the us and the eu, the requirements for medical device labeling are detailed and extensive, and may be specific. Name and place of business of manufacturer, packer or distributor. Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. What are the requirements for medical device labeling? Overall, the agency. Medical Device Labeling Requirements Font Size.
From exoptcoyn.blob.core.windows.net
Medical Device Label Font Size at Loren Pierce blog Medical Device Labeling Requirements Font Size Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. (a) the label of a device in package. Name and place of business of manufacturer, packer or distributor. What are the requirements for medical device labeling? In the us and the eu, the requirements for medical device labeling are detailed and. Medical Device Labeling Requirements Font Size.
From labelservice.co.uk
Medical Device Labels, Medical Device Labelling Labelservice Medical Device Labeling Requirements Font Size (a) the label of a device in package. Overall, the agency recommends that the font size should be no smaller than 10 points for any section of the ifu, except no. What are the requirements for medical device labeling? In the us and the eu, the requirements for medical device labeling are detailed and extensive, and may be specific. Name. Medical Device Labeling Requirements Font Size.
From clin-r.com
Labels for Medical Devices Clin R Medical Device Labeling Requirements Font Size (a) the label of a device in package. What are the requirements for medical device labeling? Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. Overall, the agency recommends that the font size should be no smaller than 10 points for any section of the ifu, except no. In the. Medical Device Labeling Requirements Font Size.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Medical Device Labeling Requirements Font Size Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. Overall, the agency recommends that the font size should be no smaller than 10 points for any section of the ifu, except no. (a) the label of a device in package. Name and place of business of manufacturer, packer or distributor.. Medical Device Labeling Requirements Font Size.
From exogmsrpv.blob.core.windows.net
Medical Device Labeling Font Size at Scott Bunger blog Medical Device Labeling Requirements Font Size What are the requirements for medical device labeling? In the us and the eu, the requirements for medical device labeling are detailed and extensive, and may be specific. Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. Name and place of business of manufacturer, packer or distributor. (a) the label. Medical Device Labeling Requirements Font Size.
From cexdbwxv.blob.core.windows.net
Fda Label Font Requirements at Toby Martinez blog Medical Device Labeling Requirements Font Size Name and place of business of manufacturer, packer or distributor. Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. (a) the label of a device in package. In the us and the eu, the requirements for medical device labeling are detailed and extensive, and may be specific. What are the. Medical Device Labeling Requirements Font Size.
From peakvascularaccess.com
What is the meaning of symbols on medical devices labels? Peak Mobile Medical Device Labeling Requirements Font Size (a) the label of a device in package. In the us and the eu, the requirements for medical device labeling are detailed and extensive, and may be specific. Name and place of business of manufacturer, packer or distributor. Overall, the agency recommends that the font size should be no smaller than 10 points for any section of the ifu, except. Medical Device Labeling Requirements Font Size.
From pharmaknowl.com
SFDA Labelling Requirements PharmaKnowl Medical Device Labeling Requirements Font Size What are the requirements for medical device labeling? Name and place of business of manufacturer, packer or distributor. Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. (a) the label of a device in package. In the us and the eu, the requirements for medical device labeling are detailed and. Medical Device Labeling Requirements Font Size.
From nextplus.io
Medical Device Labeling Compliant & UserFriendly Guide Next Plus Medical Device Labeling Requirements Font Size What are the requirements for medical device labeling? Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. Overall, the agency recommends that the font size should be no smaller than 10 points for any section of the ifu, except no. Name and place of business of manufacturer, packer or distributor.. Medical Device Labeling Requirements Font Size.
From www.scribd.com
FDA Medical Device Labeling Requirements Checklist Greenlight Guru PDF Medical Device Labeling Requirements Font Size Name and place of business of manufacturer, packer or distributor. What are the requirements for medical device labeling? (a) the label of a device in package. In the us and the eu, the requirements for medical device labeling are detailed and extensive, and may be specific. Elements of medical device and ivd medical device labelling ras require and specify information. Medical Device Labeling Requirements Font Size.
From datamyte.com
Medical Device Labeling A Comprehensive Guide DataMyte Medical Device Labeling Requirements Font Size In the us and the eu, the requirements for medical device labeling are detailed and extensive, and may be specific. What are the requirements for medical device labeling? Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. Overall, the agency recommends that the font size should be no smaller than. Medical Device Labeling Requirements Font Size.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Labeling Requirements Font Size (a) the label of a device in package. Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. Name and place of business of manufacturer, packer or distributor. What are the requirements for medical device labeling? In the us and the eu, the requirements for medical device labeling are detailed and. Medical Device Labeling Requirements Font Size.
From exogmsrpv.blob.core.windows.net
Medical Device Labeling Font Size at Scott Bunger blog Medical Device Labeling Requirements Font Size Name and place of business of manufacturer, packer or distributor. Overall, the agency recommends that the font size should be no smaller than 10 points for any section of the ifu, except no. Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. (a) the label of a device in package.. Medical Device Labeling Requirements Font Size.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Labeling Requirements Font Size In the us and the eu, the requirements for medical device labeling are detailed and extensive, and may be specific. (a) the label of a device in package. Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. What are the requirements for medical device labeling? Overall, the agency recommends that. Medical Device Labeling Requirements Font Size.
From www.slideserve.com
PPT Medical Device Labeling Requirements VISTAAR PowerPoint Medical Device Labeling Requirements Font Size (a) the label of a device in package. Name and place of business of manufacturer, packer or distributor. Overall, the agency recommends that the font size should be no smaller than 10 points for any section of the ifu, except no. What are the requirements for medical device labeling? Elements of medical device and ivd medical device labelling ras require. Medical Device Labeling Requirements Font Size.
From clin-r.com
Labels for Medical Devices Clin R Medical Device Labeling Requirements Font Size (a) the label of a device in package. Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. Overall, the agency recommends that the font size should be no smaller than 10 points for any section of the ifu, except no. What are the requirements for medical device labeling? Name and. Medical Device Labeling Requirements Font Size.
From www.techsollifesciences.com
EU MDR & IVDR Medical Device Labelling Requirements Medical Device Labeling Requirements Font Size What are the requirements for medical device labeling? Overall, the agency recommends that the font size should be no smaller than 10 points for any section of the ifu, except no. (a) the label of a device in package. Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. In the. Medical Device Labeling Requirements Font Size.
From exogmsrpv.blob.core.windows.net
Medical Device Labeling Font Size at Scott Bunger blog Medical Device Labeling Requirements Font Size In the us and the eu, the requirements for medical device labeling are detailed and extensive, and may be specific. (a) the label of a device in package. Overall, the agency recommends that the font size should be no smaller than 10 points for any section of the ifu, except no. Name and place of business of manufacturer, packer or. Medical Device Labeling Requirements Font Size.
From www.slideserve.com
PPT Medical Device Labeling Requirements VISTAAR PowerPoint Medical Device Labeling Requirements Font Size Overall, the agency recommends that the font size should be no smaller than 10 points for any section of the ifu, except no. (a) the label of a device in package. In the us and the eu, the requirements for medical device labeling are detailed and extensive, and may be specific. Elements of medical device and ivd medical device labelling. Medical Device Labeling Requirements Font Size.
From www.tailoredlabel.com
UDI Label Requirements For FDA Medical Device Labels TLP Medical Device Labeling Requirements Font Size In the us and the eu, the requirements for medical device labeling are detailed and extensive, and may be specific. (a) the label of a device in package. Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. Overall, the agency recommends that the font size should be no smaller than. Medical Device Labeling Requirements Font Size.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Labeling Requirements Font Size In the us and the eu, the requirements for medical device labeling are detailed and extensive, and may be specific. Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. Overall, the agency recommends that the font size should be no smaller than 10 points for any section of the ifu,. Medical Device Labeling Requirements Font Size.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Labeling Requirements Font Size (a) the label of a device in package. In the us and the eu, the requirements for medical device labeling are detailed and extensive, and may be specific. What are the requirements for medical device labeling? Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. Overall, the agency recommends that. Medical Device Labeling Requirements Font Size.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Medical Device Labeling Requirements Font Size Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. (a) the label of a device in package. Overall, the agency recommends that the font size should be no smaller than 10 points for any section of the ifu, except no. Name and place of business of manufacturer, packer or distributor.. Medical Device Labeling Requirements Font Size.
From ambitiousmares.blogspot.com
34 Medical Device Label Symbols Labels Design Ideas 2020 Medical Device Labeling Requirements Font Size Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. What are the requirements for medical device labeling? Overall, the agency recommends that the font size should be no smaller than 10 points for any section of the ifu, except no. In the us and the eu, the requirements for medical. Medical Device Labeling Requirements Font Size.
From www.slideserve.com
PPT Medical Device Labeling Requirements VISTAAR PowerPoint Medical Device Labeling Requirements Font Size (a) the label of a device in package. Name and place of business of manufacturer, packer or distributor. Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. Overall, the agency recommends that the font size should be no smaller than 10 points for any section of the ifu, except no.. Medical Device Labeling Requirements Font Size.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Medical Device Labeling Requirements Font Size Name and place of business of manufacturer, packer or distributor. Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. What are the requirements for medical device labeling? In the us and the eu, the requirements for medical device labeling are detailed and extensive, and may be specific. (a) the label. Medical Device Labeling Requirements Font Size.
From exogmsrpv.blob.core.windows.net
Medical Device Labeling Font Size at Scott Bunger blog Medical Device Labeling Requirements Font Size What are the requirements for medical device labeling? Elements of medical device and ivd medical device labelling ras require and specify information that manufacturers are expected to. Name and place of business of manufacturer, packer or distributor. In the us and the eu, the requirements for medical device labeling are detailed and extensive, and may be specific. (a) the label. Medical Device Labeling Requirements Font Size.