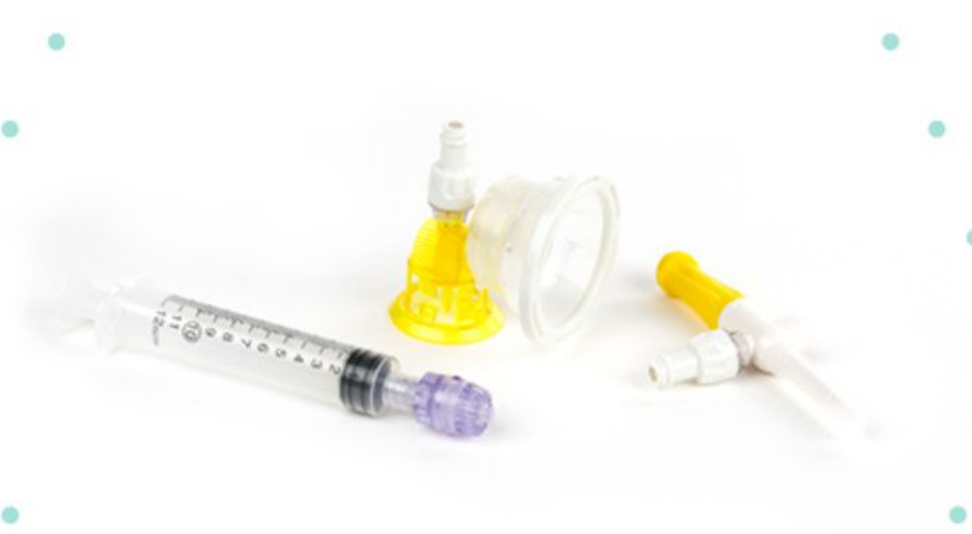
Closed System Transfer Device Usp 800 . usp <<strong>800</strong>> explicitly requires the use of closed system transfer devices (cstds) by nursing staff. usp continues to encourage early adoption and implementation of <<strong>800</strong>> to help ensure a safe environment and protection of healthcare. fred massoomi, pharmd, fashp, senior director at visante, explains how usp 800 regulations will drive the. closed system transfer devices (cstds), such as equashield’s cstd product line, play a crucial role in reducing the risk of exposure and contamination while complying with usp 800 and usp 797 guidelines. the standards set by usp chapter 800 are applicable in all settings in which hds are compounded and administered and where. usp <<strong>800</strong>> updates a previous policy in usp that allowed preparation of some hazardous drugs within a primary engineering.
from www.packagingconnections.com
closed system transfer devices (cstds), such as equashield’s cstd product line, play a crucial role in reducing the risk of exposure and contamination while complying with usp 800 and usp 797 guidelines. usp continues to encourage early adoption and implementation of <<strong>800</strong>> to help ensure a safe environment and protection of healthcare. usp <<strong>800</strong>> updates a previous policy in usp that allowed preparation of some hazardous drugs within a primary engineering. fred massoomi, pharmd, fashp, senior director at visante, explains how usp 800 regulations will drive the. usp <<strong>800</strong>> explicitly requires the use of closed system transfer devices (cstds) by nursing staff. the standards set by usp chapter 800 are applicable in all settings in which hds are compounded and administered and where.
What are CSTD aka Closed System Transfer Device & its complete system
Closed System Transfer Device Usp 800 usp <<strong>800</strong>> explicitly requires the use of closed system transfer devices (cstds) by nursing staff. usp <<strong>800</strong>> explicitly requires the use of closed system transfer devices (cstds) by nursing staff. fred massoomi, pharmd, fashp, senior director at visante, explains how usp 800 regulations will drive the. closed system transfer devices (cstds), such as equashield’s cstd product line, play a crucial role in reducing the risk of exposure and contamination while complying with usp 800 and usp 797 guidelines. the standards set by usp chapter 800 are applicable in all settings in which hds are compounded and administered and where. usp <<strong>800</strong>> updates a previous policy in usp that allowed preparation of some hazardous drugs within a primary engineering. usp continues to encourage early adoption and implementation of <<strong>800</strong>> to help ensure a safe environment and protection of healthcare.
From www.equashield.com
EQUASHIELD Efficiency of Closed System Transfer Devices Closed System Transfer Device Usp 800 the standards set by usp chapter 800 are applicable in all settings in which hds are compounded and administered and where. fred massoomi, pharmd, fashp, senior director at visante, explains how usp 800 regulations will drive the. usp continues to encourage early adoption and implementation of <<strong>800</strong>> to help ensure a safe environment and protection of healthcare.. Closed System Transfer Device Usp 800.
From www.simplivia.com
Administration of Hazardous Drugs for Nurses Simplivia Closed System Transfer Device Usp 800 usp <<strong>800</strong>> explicitly requires the use of closed system transfer devices (cstds) by nursing staff. the standards set by usp chapter 800 are applicable in all settings in which hds are compounded and administered and where. closed system transfer devices (cstds), such as equashield’s cstd product line, play a crucial role in reducing the risk of exposure. Closed System Transfer Device Usp 800.
From www.equashield.com
Closed System Transfer Device (CSTD) Solutions EQUASHIELD Closed System Transfer Device Usp 800 the standards set by usp chapter 800 are applicable in all settings in which hds are compounded and administered and where. usp <<strong>800</strong>> updates a previous policy in usp that allowed preparation of some hazardous drugs within a primary engineering. fred massoomi, pharmd, fashp, senior director at visante, explains how usp 800 regulations will drive the. . Closed System Transfer Device Usp 800.
From www.trmpo.net
ICU Medical ChemoLock 欧宝官方网站 Closed System Transfer Device Usp 800 usp <<strong>800</strong>> explicitly requires the use of closed system transfer devices (cstds) by nursing staff. fred massoomi, pharmd, fashp, senior director at visante, explains how usp 800 regulations will drive the. usp <<strong>800</strong>> updates a previous policy in usp that allowed preparation of some hazardous drugs within a primary engineering. closed system transfer devices (cstds), such. Closed System Transfer Device Usp 800.
From www.packagingconnections.com
What are CSTD aka Closed System Transfer Device & its complete system Closed System Transfer Device Usp 800 the standards set by usp chapter 800 are applicable in all settings in which hds are compounded and administered and where. usp continues to encourage early adoption and implementation of <<strong>800</strong>> to help ensure a safe environment and protection of healthcare. usp <<strong>800</strong>> explicitly requires the use of closed system transfer devices (cstds) by nursing staff. . Closed System Transfer Device Usp 800.
From www.youtube.com
EQUASHIELD®'s Closed System Transfer Device (CSTD) YouTube Closed System Transfer Device Usp 800 fred massoomi, pharmd, fashp, senior director at visante, explains how usp 800 regulations will drive the. closed system transfer devices (cstds), such as equashield’s cstd product line, play a crucial role in reducing the risk of exposure and contamination while complying with usp 800 and usp 797 guidelines. usp <<strong>800</strong>> explicitly requires the use of closed system. Closed System Transfer Device Usp 800.
From www.epic-med.com
ProSeal™ Closed System Transfer Device Closed System Transfer Device Usp 800 usp <<strong>800</strong>> updates a previous policy in usp that allowed preparation of some hazardous drugs within a primary engineering. usp continues to encourage early adoption and implementation of <<strong>800</strong>> to help ensure a safe environment and protection of healthcare. the standards set by usp chapter 800 are applicable in all settings in which hds are compounded and. Closed System Transfer Device Usp 800.
From www.pppmag.com
Closed System Transfer Device BD PP&P Magazine Pharmacy Closed System Transfer Device Usp 800 the standards set by usp chapter 800 are applicable in all settings in which hds are compounded and administered and where. usp continues to encourage early adoption and implementation of <<strong>800</strong>> to help ensure a safe environment and protection of healthcare. closed system transfer devices (cstds), such as equashield’s cstd product line, play a crucial role in. Closed System Transfer Device Usp 800.
From www.equashield.com
Product CSTD Closed Transfer System Device Equashield Closed System Transfer Device Usp 800 fred massoomi, pharmd, fashp, senior director at visante, explains how usp 800 regulations will drive the. usp <<strong>800</strong>> updates a previous policy in usp that allowed preparation of some hazardous drugs within a primary engineering. closed system transfer devices (cstds), such as equashield’s cstd product line, play a crucial role in reducing the risk of exposure and. Closed System Transfer Device Usp 800.
From www.degreec.com
USP 800 Compliance for Compounding Pharmacies & Laboratories Closed System Transfer Device Usp 800 usp <<strong>800</strong>> explicitly requires the use of closed system transfer devices (cstds) by nursing staff. usp continues to encourage early adoption and implementation of <<strong>800</strong>> to help ensure a safe environment and protection of healthcare. closed system transfer devices (cstds), such as equashield’s cstd product line, play a crucial role in reducing the risk of exposure and. Closed System Transfer Device Usp 800.
From www.equashield.com
What is a Closed System Transfer Device (CSTD)? EQUASHIELD Closed System Transfer Device Usp 800 closed system transfer devices (cstds), such as equashield’s cstd product line, play a crucial role in reducing the risk of exposure and contamination while complying with usp 800 and usp 797 guidelines. usp <<strong>800</strong>> updates a previous policy in usp that allowed preparation of some hazardous drugs within a primary engineering. usp continues to encourage early adoption. Closed System Transfer Device Usp 800.
From cmo.fresenius-kabi.com
Transfer Devices Fresenius Kabi Contract Manufacturing Closed System Transfer Device Usp 800 the standards set by usp chapter 800 are applicable in all settings in which hds are compounded and administered and where. closed system transfer devices (cstds), such as equashield’s cstd product line, play a crucial role in reducing the risk of exposure and contamination while complying with usp 800 and usp 797 guidelines. usp <<strong>800</strong>> updates a. Closed System Transfer Device Usp 800.
From www.pppmag.com
Closed System DrugTransfer Device Corvida Medical PP&P Magazine Closed System Transfer Device Usp 800 usp <<strong>800</strong>> explicitly requires the use of closed system transfer devices (cstds) by nursing staff. closed system transfer devices (cstds), such as equashield’s cstd product line, play a crucial role in reducing the risk of exposure and contamination while complying with usp 800 and usp 797 guidelines. the standards set by usp chapter 800 are applicable in. Closed System Transfer Device Usp 800.
From www.packagingconnections.com
What are CSTD aka Closed System Transfer Device & its complete system Closed System Transfer Device Usp 800 the standards set by usp chapter 800 are applicable in all settings in which hds are compounded and administered and where. usp <<strong>800</strong>> explicitly requires the use of closed system transfer devices (cstds) by nursing staff. closed system transfer devices (cstds), such as equashield’s cstd product line, play a crucial role in reducing the risk of exposure. Closed System Transfer Device Usp 800.
From www.youtube.com
How to use Qimono Closed System Transfer Device (CSTD) in IV Closed System Transfer Device Usp 800 the standards set by usp chapter 800 are applicable in all settings in which hds are compounded and administered and where. closed system transfer devices (cstds), such as equashield’s cstd product line, play a crucial role in reducing the risk of exposure and contamination while complying with usp 800 and usp 797 guidelines. fred massoomi, pharmd, fashp,. Closed System Transfer Device Usp 800.
From www.ciamedical.com
Equashield, Inc. SU35/2 Closed System Transfer Device, Syringe Unit Closed System Transfer Device Usp 800 usp continues to encourage early adoption and implementation of <<strong>800</strong>> to help ensure a safe environment and protection of healthcare. usp <<strong>800</strong>> updates a previous policy in usp that allowed preparation of some hazardous drugs within a primary engineering. the standards set by usp chapter 800 are applicable in all settings in which hds are compounded and. Closed System Transfer Device Usp 800.
From slideplayer.com
Deciphering Pharmacy Codes & ppt download Closed System Transfer Device Usp 800 usp <<strong>800</strong>> explicitly requires the use of closed system transfer devices (cstds) by nursing staff. fred massoomi, pharmd, fashp, senior director at visante, explains how usp 800 regulations will drive the. usp continues to encourage early adoption and implementation of <<strong>800</strong>> to help ensure a safe environment and protection of healthcare. the standards set by usp. Closed System Transfer Device Usp 800.
From www.bbraunusa.com
OnGuard® 2 Closed System Transfer Device Usp 800 usp <<strong>800</strong>> explicitly requires the use of closed system transfer devices (cstds) by nursing staff. fred massoomi, pharmd, fashp, senior director at visante, explains how usp 800 regulations will drive the. closed system transfer devices (cstds), such as equashield’s cstd product line, play a crucial role in reducing the risk of exposure and contamination while complying with. Closed System Transfer Device Usp 800.
From www.biophlox.com
Buy Closed System Transfer Device get price for lab equipment Closed System Transfer Device Usp 800 the standards set by usp chapter 800 are applicable in all settings in which hds are compounded and administered and where. closed system transfer devices (cstds), such as equashield’s cstd product line, play a crucial role in reducing the risk of exposure and contamination while complying with usp 800 and usp 797 guidelines. usp continues to encourage. Closed System Transfer Device Usp 800.
From psintelligence.blogspot.com
Closed System Transfer Devices Industry To Grow Fastest in AsiaPacific Closed System Transfer Device Usp 800 usp <<strong>800</strong>> updates a previous policy in usp that allowed preparation of some hazardous drugs within a primary engineering. closed system transfer devices (cstds), such as equashield’s cstd product line, play a crucial role in reducing the risk of exposure and contamination while complying with usp 800 and usp 797 guidelines. usp <<strong>800</strong>> explicitly requires the use. Closed System Transfer Device Usp 800.
From ejhp.bmj.com
Container closure integrity testing and process validation of closed Closed System Transfer Device Usp 800 usp <<strong>800</strong>> explicitly requires the use of closed system transfer devices (cstds) by nursing staff. fred massoomi, pharmd, fashp, senior director at visante, explains how usp 800 regulations will drive the. closed system transfer devices (cstds), such as equashield’s cstd product line, play a crucial role in reducing the risk of exposure and contamination while complying with. Closed System Transfer Device Usp 800.
From www.bbraunusa.com
OnGuard® 2 Closed System Transfer Device Usp 800 fred massoomi, pharmd, fashp, senior director at visante, explains how usp 800 regulations will drive the. usp <<strong>800</strong>> updates a previous policy in usp that allowed preparation of some hazardous drugs within a primary engineering. usp continues to encourage early adoption and implementation of <<strong>800</strong>> to help ensure a safe environment and protection of healthcare. usp. Closed System Transfer Device Usp 800.
From www.progressivemedinc.com
Arisure® Closed System Transfer Device Progressive Medical, Inc Closed System Transfer Device Usp 800 closed system transfer devices (cstds), such as equashield’s cstd product line, play a crucial role in reducing the risk of exposure and contamination while complying with usp 800 and usp 797 guidelines. fred massoomi, pharmd, fashp, senior director at visante, explains how usp 800 regulations will drive the. usp continues to encourage early adoption and implementation of. Closed System Transfer Device Usp 800.
From www.youtube.com
easyFlow Closed Transfer System YouTube Closed System Transfer Device Usp 800 the standards set by usp chapter 800 are applicable in all settings in which hds are compounded and administered and where. fred massoomi, pharmd, fashp, senior director at visante, explains how usp 800 regulations will drive the. usp continues to encourage early adoption and implementation of <<strong>800</strong>> to help ensure a safe environment and protection of healthcare.. Closed System Transfer Device Usp 800.
From www.simplivia.com
Closed System Drug Transfer Devices (CSTD) Simplivia Closed System Transfer Device Usp 800 usp <<strong>800</strong>> explicitly requires the use of closed system transfer devices (cstds) by nursing staff. usp continues to encourage early adoption and implementation of <<strong>800</strong>> to help ensure a safe environment and protection of healthcare. closed system transfer devices (cstds), such as equashield’s cstd product line, play a crucial role in reducing the risk of exposure and. Closed System Transfer Device Usp 800.
From www.ndc-inc.com
OnGuard® Closed System Transfer Device (CSTD) Closed System Transfer Device Usp 800 usp continues to encourage early adoption and implementation of <<strong>800</strong>> to help ensure a safe environment and protection of healthcare. fred massoomi, pharmd, fashp, senior director at visante, explains how usp 800 regulations will drive the. closed system transfer devices (cstds), such as equashield’s cstd product line, play a crucial role in reducing the risk of exposure. Closed System Transfer Device Usp 800.
From www.pppmag.com
Closed System Transfer Device Allison Medical, Inc PP&P Magazine Closed System Transfer Device Usp 800 closed system transfer devices (cstds), such as equashield’s cstd product line, play a crucial role in reducing the risk of exposure and contamination while complying with usp 800 and usp 797 guidelines. usp <<strong>800</strong>> explicitly requires the use of closed system transfer devices (cstds) by nursing staff. the standards set by usp chapter 800 are applicable in. Closed System Transfer Device Usp 800.
From www.youtube.com
Halo Closed System Transfer Device (CSTD) YouTube Closed System Transfer Device Usp 800 closed system transfer devices (cstds), such as equashield’s cstd product line, play a crucial role in reducing the risk of exposure and contamination while complying with usp 800 and usp 797 guidelines. fred massoomi, pharmd, fashp, senior director at visante, explains how usp 800 regulations will drive the. usp continues to encourage early adoption and implementation of. Closed System Transfer Device Usp 800.
From www.frontiersin.org
Frontiers Evaluation of Closed System Transfer Devices in Preventing Closed System Transfer Device Usp 800 usp <<strong>800</strong>> explicitly requires the use of closed system transfer devices (cstds) by nursing staff. the standards set by usp chapter 800 are applicable in all settings in which hds are compounded and administered and where. usp <<strong>800</strong>> updates a previous policy in usp that allowed preparation of some hazardous drugs within a primary engineering. usp. Closed System Transfer Device Usp 800.
From www.healthynewage.com
Increasing adoption of chemotherapy in cancer treatments fosters demand Closed System Transfer Device Usp 800 usp <<strong>800</strong>> updates a previous policy in usp that allowed preparation of some hazardous drugs within a primary engineering. usp continues to encourage early adoption and implementation of <<strong>800</strong>> to help ensure a safe environment and protection of healthcare. usp <<strong>800</strong>> explicitly requires the use of closed system transfer devices (cstds) by nursing staff. the standards. Closed System Transfer Device Usp 800.
From www.graylinemedical.com
ICU Medical ChemoLock Closed System Transfer Device ChemoLock Vented Closed System Transfer Device Usp 800 usp <<strong>800</strong>> explicitly requires the use of closed system transfer devices (cstds) by nursing staff. usp continues to encourage early adoption and implementation of <<strong>800</strong>> to help ensure a safe environment and protection of healthcare. usp <<strong>800</strong>> updates a previous policy in usp that allowed preparation of some hazardous drugs within a primary engineering. fred massoomi,. Closed System Transfer Device Usp 800.
From www.bbrauneuniversity.com
Closed System Drug Transfer Device Closed System Transfer Device Usp 800 the standards set by usp chapter 800 are applicable in all settings in which hds are compounded and administered and where. fred massoomi, pharmd, fashp, senior director at visante, explains how usp 800 regulations will drive the. closed system transfer devices (cstds), such as equashield’s cstd product line, play a crucial role in reducing the risk of. Closed System Transfer Device Usp 800.
From www.equashield.com
Comparing The Efficiency of Closed System Transfer Devices for Closed System Transfer Device Usp 800 usp <<strong>800</strong>> explicitly requires the use of closed system transfer devices (cstds) by nursing staff. the standards set by usp chapter 800 are applicable in all settings in which hds are compounded and administered and where. usp <<strong>800</strong>> updates a previous policy in usp that allowed preparation of some hazardous drugs within a primary engineering. fred. Closed System Transfer Device Usp 800.
From todaysveterinarybusiness.com
Are you ready for USP 800? Today's Veterinary Business Closed System Transfer Device Usp 800 usp <<strong>800</strong>> explicitly requires the use of closed system transfer devices (cstds) by nursing staff. closed system transfer devices (cstds), such as equashield’s cstd product line, play a crucial role in reducing the risk of exposure and contamination while complying with usp 800 and usp 797 guidelines. usp <<strong>800</strong>> updates a previous policy in usp that allowed. Closed System Transfer Device Usp 800.
From www.pppmag.com
Closed System Transfer Device Equashield Compounding Technologies Closed System Transfer Device Usp 800 usp <<strong>800</strong>> updates a previous policy in usp that allowed preparation of some hazardous drugs within a primary engineering. usp <<strong>800</strong>> explicitly requires the use of closed system transfer devices (cstds) by nursing staff. closed system transfer devices (cstds), such as equashield’s cstd product line, play a crucial role in reducing the risk of exposure and contamination. Closed System Transfer Device Usp 800.