How Does Molarity Affect Cell Potential . It relates the measured cell. The cell potential is the potential an electron experiences; Eº cell is the standard state cell potential, which means that the value was determined. This dependence of the cell potential on the. This flow of charged particles is. Cell potential definitely depends on the concentration of the two solutions. Students use the concept of water potential to determine how water will move into or out of the cell. The potential difference is caused by the ability of electrons to flow from one half cell to the other. Describe and relate the definitions of electrode and cell potentials. Electrons are able to move between electrodes because the chemical reaction. Interpret electrode potentials in terms of relative oxidant and reductant. Why is e $^\circ_\mathrm{cell}$ independent of molar coefficients (3,2)? Difference between e cell and eº cell. The first page provides background information and definitions for. Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons.
from chart-studio.plotly.com
The potential difference is caused by the ability of electrons to flow from one half cell to the other. Interpret electrode potentials in terms of relative oxidant and reductant. Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. This flow of charged particles is. The cell potential is the potential an electron experiences; The first page provides background information and definitions for. Difference between e cell and eº cell. Describe and relate the definitions of electrode and cell potentials. Eº cell is the standard state cell potential, which means that the value was determined. It relates the measured cell.
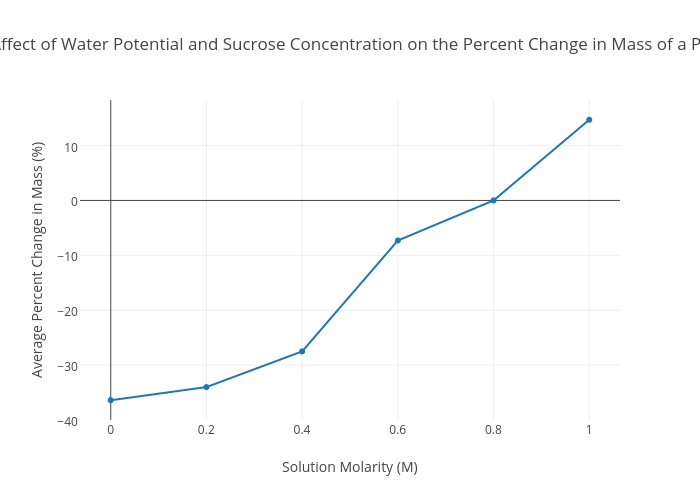
The Affect of Water Potential and Sucrose Concentration on the Percent
How Does Molarity Affect Cell Potential It relates the measured cell. This dependence of the cell potential on the. Electrons are able to move between electrodes because the chemical reaction. Difference between e cell and eº cell. Describe and relate the definitions of electrode and cell potentials. Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. Students use the concept of water potential to determine how water will move into or out of the cell. Eº cell is the standard state cell potential, which means that the value was determined. The potential difference is caused by the ability of electrons to flow from one half cell to the other. Why is e $^\circ_\mathrm{cell}$ independent of molar coefficients (3,2)? The cell potential is the potential an electron experiences; This flow of charged particles is. The first page provides background information and definitions for. Cell potential definitely depends on the concentration of the two solutions. It relates the measured cell. Interpret electrode potentials in terms of relative oxidant and reductant.
From www.youtube.com
DILUTION AND MIXING OF SOLUTIONS, MOLARITY EQUATION /SOME BASIC How Does Molarity Affect Cell Potential Students use the concept of water potential to determine how water will move into or out of the cell. Electrons are able to move between electrodes because the chemical reaction. Cell potential definitely depends on the concentration of the two solutions. The cell potential is the potential an electron experiences; The first page provides background information and definitions for. Electrochemical. How Does Molarity Affect Cell Potential.
From wou.edu
CH104 Chapter 7 Solutions Chemistry How Does Molarity Affect Cell Potential Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. Electrons are able to move between electrodes because the chemical reaction. The cell potential is the potential an electron experiences; This dependence of the cell potential on the. Interpret electrode potentials in terms of relative oxidant and reductant. This flow of charged particles is.. How Does Molarity Affect Cell Potential.
From www.youtube.com
Molarity, Molality, Volume & Mass Percent, Mole Fraction & Density How Does Molarity Affect Cell Potential Eº cell is the standard state cell potential, which means that the value was determined. It relates the measured cell. Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. The potential difference is caused by the ability of electrons to flow from one half cell to the other. This flow of charged particles. How Does Molarity Affect Cell Potential.
From www.youtube.com
Chemistry Basics Osmolarity, Osmolality and Tonicity YouTube How Does Molarity Affect Cell Potential Cell potential definitely depends on the concentration of the two solutions. The first page provides background information and definitions for. The cell potential is the potential an electron experiences; Why is e $^\circ_\mathrm{cell}$ independent of molar coefficients (3,2)? Describe and relate the definitions of electrode and cell potentials. Eº cell is the standard state cell potential, which means that the. How Does Molarity Affect Cell Potential.
From slideplayer.com
Electrochemistry. ppt download How Does Molarity Affect Cell Potential This dependence of the cell potential on the. Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. Students use the concept of water potential to determine how water will move into or out of the cell. The potential difference is caused by the ability of electrons to flow from one half cell to. How Does Molarity Affect Cell Potential.
From slideplayer.com
Resource Acquisition and Transport in Vascular Plants ppt download How Does Molarity Affect Cell Potential Interpret electrode potentials in terms of relative oxidant and reductant. Describe and relate the definitions of electrode and cell potentials. Eº cell is the standard state cell potential, which means that the value was determined. This dependence of the cell potential on the. Students use the concept of water potential to determine how water will move into or out of. How Does Molarity Affect Cell Potential.
From inspiritvr.com
Molarity Study Guide Inspirit How Does Molarity Affect Cell Potential The potential difference is caused by the ability of electrons to flow from one half cell to the other. Interpret electrode potentials in terms of relative oxidant and reductant. Difference between e cell and eº cell. It relates the measured cell. This flow of charged particles is. Eº cell is the standard state cell potential, which means that the value. How Does Molarity Affect Cell Potential.
From byjus.com
relation between molarity,molality,and density of solution How Does Molarity Affect Cell Potential Why is e $^\circ_\mathrm{cell}$ independent of molar coefficients (3,2)? Difference between e cell and eº cell. The potential difference is caused by the ability of electrons to flow from one half cell to the other. Interpret electrode potentials in terms of relative oxidant and reductant. Electrons are able to move between electrodes because the chemical reaction. Electrochemical cells use redox. How Does Molarity Affect Cell Potential.
From general.chemistrysteps.com
Stoichiometry of Chemical Reactions Chemistry Steps How Does Molarity Affect Cell Potential Students use the concept of water potential to determine how water will move into or out of the cell. Why is e $^\circ_\mathrm{cell}$ independent of molar coefficients (3,2)? Electrons are able to move between electrodes because the chemical reaction. This dependence of the cell potential on the. Eº cell is the standard state cell potential, which means that the value. How Does Molarity Affect Cell Potential.
From courses.lumenlearning.com
Colligative Properties Chemistry Atoms First How Does Molarity Affect Cell Potential Describe and relate the definitions of electrode and cell potentials. Students use the concept of water potential to determine how water will move into or out of the cell. The cell potential is the potential an electron experiences; Electrons are able to move between electrodes because the chemical reaction. This flow of charged particles is. Interpret electrode potentials in terms. How Does Molarity Affect Cell Potential.
From www.slideserve.com
PPT Molality PowerPoint Presentation, free download ID4499739 How Does Molarity Affect Cell Potential Eº cell is the standard state cell potential, which means that the value was determined. The first page provides background information and definitions for. Cell potential definitely depends on the concentration of the two solutions. The cell potential is the potential an electron experiences; Why is e $^\circ_\mathrm{cell}$ independent of molar coefficients (3,2)? Difference between e cell and eº cell.. How Does Molarity Affect Cell Potential.
From www.studocu.com
Dilutions, Molarity and Titrations Cell Biology and Biochemistry How Does Molarity Affect Cell Potential Interpret electrode potentials in terms of relative oxidant and reductant. Why is e $^\circ_\mathrm{cell}$ independent of molar coefficients (3,2)? Cell potential definitely depends on the concentration of the two solutions. The first page provides background information and definitions for. Electrons are able to move between electrodes because the chemical reaction. The cell potential is the potential an electron experiences; Describe. How Does Molarity Affect Cell Potential.
From www.youtube.com
Detemining E cell when the concentrations are not 1 molar using Nernst How Does Molarity Affect Cell Potential The potential difference is caused by the ability of electrons to flow from one half cell to the other. Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. Electrons are able to move between electrodes because the chemical reaction. The cell potential is the potential an electron experiences; It relates the measured cell.. How Does Molarity Affect Cell Potential.
From halleldmoses.blogspot.com
Cell Potential Formula HalleldMoses How Does Molarity Affect Cell Potential It relates the measured cell. Difference between e cell and eº cell. The cell potential is the potential an electron experiences; Describe and relate the definitions of electrode and cell potentials. This flow of charged particles is. This dependence of the cell potential on the. Cell potential definitely depends on the concentration of the two solutions. The first page provides. How Does Molarity Affect Cell Potential.
From www.numerade.com
SOLVED EXPERIMENT 2 The initial molarity of the Cu2+ and Zn2 How Does Molarity Affect Cell Potential Why is e $^\circ_\mathrm{cell}$ independent of molar coefficients (3,2)? The first page provides background information and definitions for. Describe and relate the definitions of electrode and cell potentials. Electrons are able to move between electrodes because the chemical reaction. Students use the concept of water potential to determine how water will move into or out of the cell. Electrochemical cells. How Does Molarity Affect Cell Potential.
From www.tes.com
Molar Volume of Gases GCSE Lesson (SC14e) TRIPLE Teaching Resources How Does Molarity Affect Cell Potential Why is e $^\circ_\mathrm{cell}$ independent of molar coefficients (3,2)? The cell potential is the potential an electron experiences; This dependence of the cell potential on the. Describe and relate the definitions of electrode and cell potentials. The potential difference is caused by the ability of electrons to flow from one half cell to the other. Eº cell is the standard. How Does Molarity Affect Cell Potential.
From socratic.org
How does concentration affect galvanic cell? Socratic How Does Molarity Affect Cell Potential Cell potential definitely depends on the concentration of the two solutions. The potential difference is caused by the ability of electrons to flow from one half cell to the other. Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. This dependence of the cell potential on the. Electrons are able to move between. How Does Molarity Affect Cell Potential.
From www.slideserve.com
PPT Molarity and Molality PowerPoint Presentation, free download ID How Does Molarity Affect Cell Potential Why is e $^\circ_\mathrm{cell}$ independent of molar coefficients (3,2)? Cell potential definitely depends on the concentration of the two solutions. Interpret electrode potentials in terms of relative oxidant and reductant. Eº cell is the standard state cell potential, which means that the value was determined. It relates the measured cell. Students use the concept of water potential to determine how. How Does Molarity Affect Cell Potential.
From slideplayer.com
What is Water Potential? ppt download How Does Molarity Affect Cell Potential Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and reductant. The potential difference is caused by the ability of electrons to flow from one half cell to the other. This flow of charged particles is. This dependence of the cell potential on the. It relates the measured cell. The first. How Does Molarity Affect Cell Potential.
From mungfali.com
Nernst Equation Cell Potential How Does Molarity Affect Cell Potential The potential difference is caused by the ability of electrons to flow from one half cell to the other. Students use the concept of water potential to determine how water will move into or out of the cell. Difference between e cell and eº cell. Electrons are able to move between electrodes because the chemical reaction. The cell potential is. How Does Molarity Affect Cell Potential.
From www.youtube.com
Ion Concentration in Solutions From Molarity, Chemistry Practice How Does Molarity Affect Cell Potential The first page provides background information and definitions for. Why is e $^\circ_\mathrm{cell}$ independent of molar coefficients (3,2)? This flow of charged particles is. This dependence of the cell potential on the. It relates the measured cell. Electrons are able to move between electrodes because the chemical reaction. The cell potential is the potential an electron experiences; Cell potential definitely. How Does Molarity Affect Cell Potential.
From www.slideserve.com
PPT Concentration PowerPoint Presentation, free download ID2186169 How Does Molarity Affect Cell Potential Electrons are able to move between electrodes because the chemical reaction. Interpret electrode potentials in terms of relative oxidant and reductant. The cell potential is the potential an electron experiences; Why is e $^\circ_\mathrm{cell}$ independent of molar coefficients (3,2)? The potential difference is caused by the ability of electrons to flow from one half cell to the other. Difference between. How Does Molarity Affect Cell Potential.
From www.learntocalculate.com
How to Calculate Cell Potential. How Does Molarity Affect Cell Potential Describe and relate the definitions of electrode and cell potentials. It relates the measured cell. Students use the concept of water potential to determine how water will move into or out of the cell. Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. The cell potential is the potential an electron experiences; Why. How Does Molarity Affect Cell Potential.
From www.youtube.com
Molarity Chemistry Tutorial YouTube How Does Molarity Affect Cell Potential The first page provides background information and definitions for. Why is e $^\circ_\mathrm{cell}$ independent of molar coefficients (3,2)? The cell potential is the potential an electron experiences; Electrons are able to move between electrodes because the chemical reaction. Cell potential definitely depends on the concentration of the two solutions. This flow of charged particles is. This dependence of the cell. How Does Molarity Affect Cell Potential.
From www.slideserve.com
PPT Water Potential PowerPoint Presentation, free download ID6323159 How Does Molarity Affect Cell Potential Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. Eº cell is the standard state cell potential, which means that the value was determined. Why is e $^\circ_\mathrm{cell}$ independent of molar coefficients (3,2)? Students use the concept of water potential to determine how water will move into or out of the cell. The. How Does Molarity Affect Cell Potential.
From www.slideserve.com
PPT Molarity PowerPoint Presentation, free download ID6829155 How Does Molarity Affect Cell Potential Difference between e cell and eº cell. The potential difference is caused by the ability of electrons to flow from one half cell to the other. This flow of charged particles is. Why is e $^\circ_\mathrm{cell}$ independent of molar coefficients (3,2)? The cell potential is the potential an electron experiences; This dependence of the cell potential on the. Cell potential. How Does Molarity Affect Cell Potential.
From www.acs.org
Molarity American Chemical Society How Does Molarity Affect Cell Potential Describe and relate the definitions of electrode and cell potentials. Cell potential definitely depends on the concentration of the two solutions. This flow of charged particles is. Interpret electrode potentials in terms of relative oxidant and reductant. Students use the concept of water potential to determine how water will move into or out of the cell. Why is e $^\circ_\mathrm{cell}$. How Does Molarity Affect Cell Potential.
From www.youtube.com
Standard Cell Potentials YouTube How Does Molarity Affect Cell Potential The cell potential is the potential an electron experiences; This dependence of the cell potential on the. Electrons are able to move between electrodes because the chemical reaction. The first page provides background information and definitions for. The potential difference is caused by the ability of electrons to flow from one half cell to the other. Why is e $^\circ_\mathrm{cell}$. How Does Molarity Affect Cell Potential.
From slideplayer.com
Membranes Chapter ppt download How Does Molarity Affect Cell Potential Cell potential definitely depends on the concentration of the two solutions. Eº cell is the standard state cell potential, which means that the value was determined. Describe and relate the definitions of electrode and cell potentials. Electrons are able to move between electrodes because the chemical reaction. The potential difference is caused by the ability of electrons to flow from. How Does Molarity Affect Cell Potential.
From slideplayer.com
Membrane Structure and Function ppt download How Does Molarity Affect Cell Potential The cell potential is the potential an electron experiences; It relates the measured cell. Cell potential definitely depends on the concentration of the two solutions. Electrons are able to move between electrodes because the chemical reaction. The potential difference is caused by the ability of electrons to flow from one half cell to the other. Students use the concept of. How Does Molarity Affect Cell Potential.
From www.wikihow.com
Molarity Formula How to Calculate Molarity with Examples How Does Molarity Affect Cell Potential This flow of charged particles is. This dependence of the cell potential on the. Interpret electrode potentials in terms of relative oxidant and reductant. Describe and relate the definitions of electrode and cell potentials. Why is e $^\circ_\mathrm{cell}$ independent of molar coefficients (3,2)? Students use the concept of water potential to determine how water will move into or out of. How Does Molarity Affect Cell Potential.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free How Does Molarity Affect Cell Potential Describe and relate the definitions of electrode and cell potentials. Eº cell is the standard state cell potential, which means that the value was determined. Electrons are able to move between electrodes because the chemical reaction. This flow of charged particles is. Why is e $^\circ_\mathrm{cell}$ independent of molar coefficients (3,2)? The cell potential is the potential an electron experiences;. How Does Molarity Affect Cell Potential.
From www.vrogue.co
Standard Reduction Potentials Redox Reactions And Ele vrogue.co How Does Molarity Affect Cell Potential Describe and relate the definitions of electrode and cell potentials. Difference between e cell and eº cell. It relates the measured cell. Students use the concept of water potential to determine how water will move into or out of the cell. This dependence of the cell potential on the. Why is e $^\circ_\mathrm{cell}$ independent of molar coefficients (3,2)? Interpret electrode. How Does Molarity Affect Cell Potential.
From www.slideserve.com
PPT Membrane Dynamics Movement and Potential PowerPoint Presentation How Does Molarity Affect Cell Potential Describe and relate the definitions of electrode and cell potentials. Why is e $^\circ_\mathrm{cell}$ independent of molar coefficients (3,2)? It relates the measured cell. Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. This flow of charged particles is. The first page provides background information and definitions for. Students use the concept of. How Does Molarity Affect Cell Potential.
From chart-studio.plotly.com
The Affect of Water Potential and Sucrose Concentration on the Percent How Does Molarity Affect Cell Potential This dependence of the cell potential on the. Interpret electrode potentials in terms of relative oxidant and reductant. Describe and relate the definitions of electrode and cell potentials. Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. Students use the concept of water potential to determine how water will move into or out. How Does Molarity Affect Cell Potential.