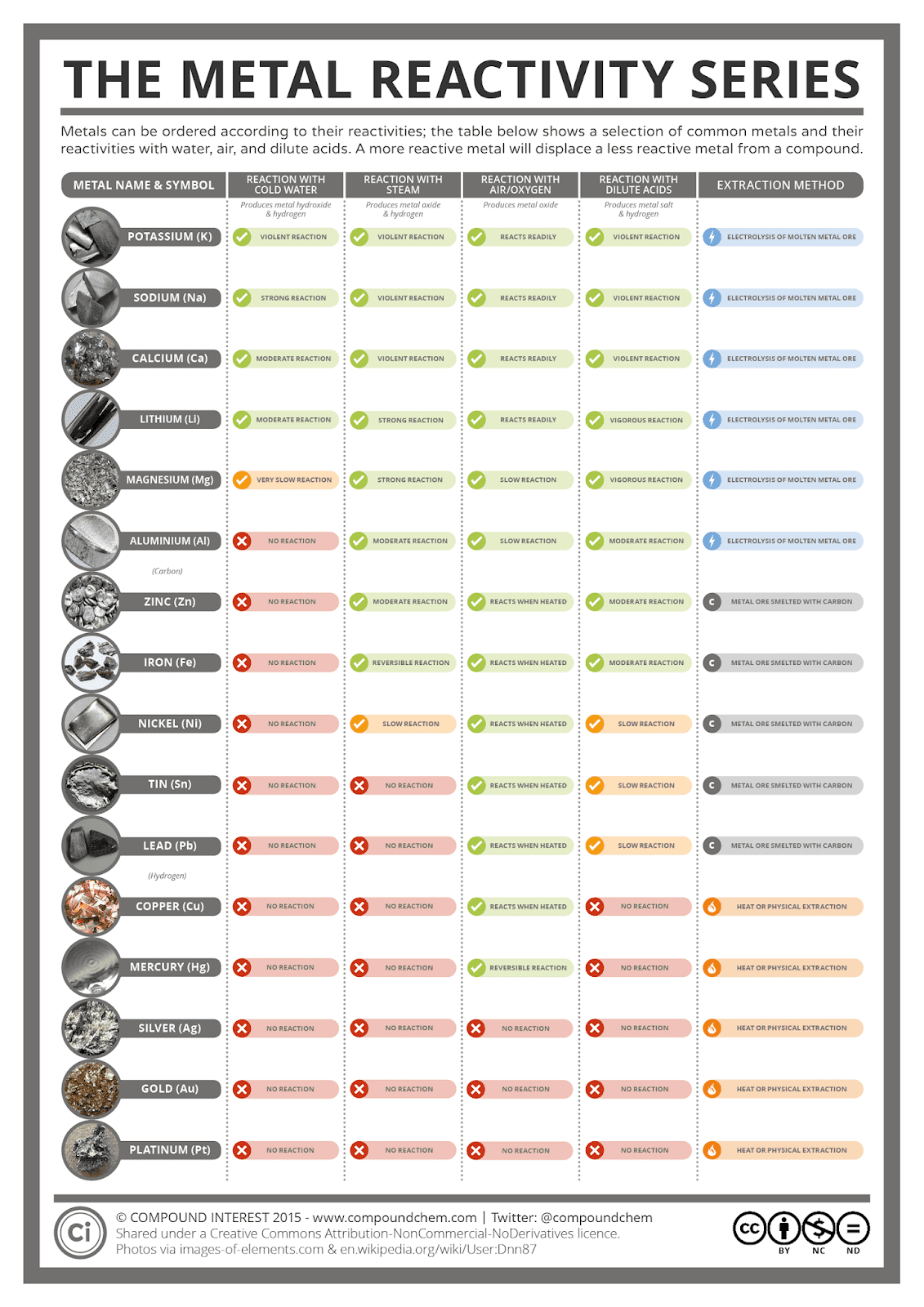
How Does The Electrochemical Series Work . the electrochemical series, also referred to as the activity series, is the ranking of metals organized by standard electrode potentials. this page explains the origin of the electrochemical series, and shows how it can be used to work out the ability of the. The standard electrode potential is the. in the electrochemical series, metals and other substances are arranged in decreasing order of their tendency to gain. the electrochemical series tells us how electropositive or electronegative the element/ion combination is, compared to standard hydrogen electrode. importance and applications of electrochemical series (with examples) 1) for comparison of the relative oxidizing. electrochemical series, also referred to as activity series, is a list that describes the arrangement of elements in the order of their increasing electrode potential.
from kanayatichemistry.blogspot.com
this page explains the origin of the electrochemical series, and shows how it can be used to work out the ability of the. the electrochemical series, also referred to as the activity series, is the ranking of metals organized by standard electrode potentials. importance and applications of electrochemical series (with examples) 1) for comparison of the relative oxidizing. in the electrochemical series, metals and other substances are arranged in decreasing order of their tendency to gain. electrochemical series, also referred to as activity series, is a list that describes the arrangement of elements in the order of their increasing electrode potential. the electrochemical series tells us how electropositive or electronegative the element/ion combination is, compared to standard hydrogen electrode. The standard electrode potential is the.
Reactivity series table K Chemistry
How Does The Electrochemical Series Work The standard electrode potential is the. the electrochemical series tells us how electropositive or electronegative the element/ion combination is, compared to standard hydrogen electrode. importance and applications of electrochemical series (with examples) 1) for comparison of the relative oxidizing. The standard electrode potential is the. the electrochemical series, also referred to as the activity series, is the ranking of metals organized by standard electrode potentials. in the electrochemical series, metals and other substances are arranged in decreasing order of their tendency to gain. this page explains the origin of the electrochemical series, and shows how it can be used to work out the ability of the. electrochemical series, also referred to as activity series, is a list that describes the arrangement of elements in the order of their increasing electrode potential.
From www.teachmint.com
Electrochemical Series Physical Chemistry Notes Teachmint How Does The Electrochemical Series Work importance and applications of electrochemical series (with examples) 1) for comparison of the relative oxidizing. the electrochemical series tells us how electropositive or electronegative the element/ion combination is, compared to standard hydrogen electrode. The standard electrode potential is the. the electrochemical series, also referred to as the activity series, is the ranking of metals organized by standard. How Does The Electrochemical Series Work.
From getrevising.co.uk
The Reactivity Series Revision Notes in GCSE Chemistry How Does The Electrochemical Series Work this page explains the origin of the electrochemical series, and shows how it can be used to work out the ability of the. the electrochemical series tells us how electropositive or electronegative the element/ion combination is, compared to standard hydrogen electrode. electrochemical series, also referred to as activity series, is a list that describes the arrangement of. How Does The Electrochemical Series Work.
From quizizz.com
Reactivity Series Chemistry Quiz Quizizz How Does The Electrochemical Series Work this page explains the origin of the electrochemical series, and shows how it can be used to work out the ability of the. importance and applications of electrochemical series (with examples) 1) for comparison of the relative oxidizing. The standard electrode potential is the. the electrochemical series, also referred to as the activity series, is the ranking. How Does The Electrochemical Series Work.
From www.researchgate.net
4 Electrochemical series. Facts from table 1. More electropositive... Download Scientific How Does The Electrochemical Series Work The standard electrode potential is the. in the electrochemical series, metals and other substances are arranged in decreasing order of their tendency to gain. this page explains the origin of the electrochemical series, and shows how it can be used to work out the ability of the. electrochemical series, also referred to as activity series, is a. How Does The Electrochemical Series Work.
From saylordotorg.github.io
Electrochemistry How Does The Electrochemical Series Work the electrochemical series, also referred to as the activity series, is the ranking of metals organized by standard electrode potentials. this page explains the origin of the electrochemical series, and shows how it can be used to work out the ability of the. importance and applications of electrochemical series (with examples) 1) for comparison of the relative. How Does The Electrochemical Series Work.
From www.vrogue.co
Reactivity Series Of Metals Chem Notes In 2020 Chemis vrogue.co How Does The Electrochemical Series Work the electrochemical series tells us how electropositive or electronegative the element/ion combination is, compared to standard hydrogen electrode. in the electrochemical series, metals and other substances are arranged in decreasing order of their tendency to gain. electrochemical series, also referred to as activity series, is a list that describes the arrangement of elements in the order of. How Does The Electrochemical Series Work.
From saylordotorg.github.io
Electrochemistry How Does The Electrochemical Series Work The standard electrode potential is the. electrochemical series, also referred to as activity series, is a list that describes the arrangement of elements in the order of their increasing electrode potential. this page explains the origin of the electrochemical series, and shows how it can be used to work out the ability of the. importance and applications. How Does The Electrochemical Series Work.
From www.youtube.com
What Is The Electrochemical Series Reactions Chemistry FuseSchool YouTube How Does The Electrochemical Series Work this page explains the origin of the electrochemical series, and shows how it can be used to work out the ability of the. importance and applications of electrochemical series (with examples) 1) for comparison of the relative oxidizing. electrochemical series, also referred to as activity series, is a list that describes the arrangement of elements in the. How Does The Electrochemical Series Work.
From www.chemistrylearner.com
Reactivity Series Definition and Chart How Does The Electrochemical Series Work the electrochemical series tells us how electropositive or electronegative the element/ion combination is, compared to standard hydrogen electrode. importance and applications of electrochemical series (with examples) 1) for comparison of the relative oxidizing. in the electrochemical series, metals and other substances are arranged in decreasing order of their tendency to gain. the electrochemical series, also referred. How Does The Electrochemical Series Work.
From study.com
Electrochemical Series Overview, Uses & Examples Lesson How Does The Electrochemical Series Work importance and applications of electrochemical series (with examples) 1) for comparison of the relative oxidizing. electrochemical series, also referred to as activity series, is a list that describes the arrangement of elements in the order of their increasing electrode potential. in the electrochemical series, metals and other substances are arranged in decreasing order of their tendency to. How Does The Electrochemical Series Work.
From svpwiki.com
Sympathetic Vibratory Physics Electromotive Series How Does The Electrochemical Series Work the electrochemical series, also referred to as the activity series, is the ranking of metals organized by standard electrode potentials. in the electrochemical series, metals and other substances are arranged in decreasing order of their tendency to gain. The standard electrode potential is the. electrochemical series, also referred to as activity series, is a list that describes. How Does The Electrochemical Series Work.
From www.dynamicscience.com.au
redox reactions electrolysis The difference between electrochemical and electrolysis. How Does The Electrochemical Series Work The standard electrode potential is the. the electrochemical series, also referred to as the activity series, is the ranking of metals organized by standard electrode potentials. electrochemical series, also referred to as activity series, is a list that describes the arrangement of elements in the order of their increasing electrode potential. the electrochemical series tells us how. How Does The Electrochemical Series Work.
From leverageedu.com
Electrochemical Series Notes Chemistry Class 11 & 12 Leverage Edu How Does The Electrochemical Series Work importance and applications of electrochemical series (with examples) 1) for comparison of the relative oxidizing. this page explains the origin of the electrochemical series, and shows how it can be used to work out the ability of the. the electrochemical series, also referred to as the activity series, is the ranking of metals organized by standard electrode. How Does The Electrochemical Series Work.
From www.studypage.in
Electrochemical Series Study Page How Does The Electrochemical Series Work electrochemical series, also referred to as activity series, is a list that describes the arrangement of elements in the order of their increasing electrode potential. the electrochemical series tells us how electropositive or electronegative the element/ion combination is, compared to standard hydrogen electrode. this page explains the origin of the electrochemical series, and shows how it can. How Does The Electrochemical Series Work.
From www.vrogue.co
What Is A Reactivity Series How Does The Reactivity S vrogue.co How Does The Electrochemical Series Work electrochemical series, also referred to as activity series, is a list that describes the arrangement of elements in the order of their increasing electrode potential. this page explains the origin of the electrochemical series, and shows how it can be used to work out the ability of the. the electrochemical series, also referred to as the activity. How Does The Electrochemical Series Work.
From scienceprojects.lv
Electrochemical series How Does The Electrochemical Series Work this page explains the origin of the electrochemical series, and shows how it can be used to work out the ability of the. The standard electrode potential is the. the electrochemical series tells us how electropositive or electronegative the element/ion combination is, compared to standard hydrogen electrode. in the electrochemical series, metals and other substances are arranged. How Does The Electrochemical Series Work.
From sciencenotes.org
Activity Series of Metals (Reactivity Series) How Does The Electrochemical Series Work The standard electrode potential is the. importance and applications of electrochemical series (with examples) 1) for comparison of the relative oxidizing. the electrochemical series tells us how electropositive or electronegative the element/ion combination is, compared to standard hydrogen electrode. electrochemical series, also referred to as activity series, is a list that describes the arrangement of elements in. How Does The Electrochemical Series Work.
From revisechemistry.uk
Reactivity of Metals AQA C4 revisechemistry.uk How Does The Electrochemical Series Work the electrochemical series tells us how electropositive or electronegative the element/ion combination is, compared to standard hydrogen electrode. this page explains the origin of the electrochemical series, and shows how it can be used to work out the ability of the. importance and applications of electrochemical series (with examples) 1) for comparison of the relative oxidizing. . How Does The Electrochemical Series Work.
From www.chemistrylearner.com
Electrochemical Series Definition, Chart, And Applications How Does The Electrochemical Series Work importance and applications of electrochemical series (with examples) 1) for comparison of the relative oxidizing. in the electrochemical series, metals and other substances are arranged in decreasing order of their tendency to gain. this page explains the origin of the electrochemical series, and shows how it can be used to work out the ability of the. . How Does The Electrochemical Series Work.
From byjus.com
How to learn the electrochemical series How Does The Electrochemical Series Work The standard electrode potential is the. this page explains the origin of the electrochemical series, and shows how it can be used to work out the ability of the. the electrochemical series tells us how electropositive or electronegative the element/ion combination is, compared to standard hydrogen electrode. importance and applications of electrochemical series (with examples) 1) for. How Does The Electrochemical Series Work.
From byjus.com
1. Electrochemical series How Does The Electrochemical Series Work electrochemical series, also referred to as activity series, is a list that describes the arrangement of elements in the order of their increasing electrode potential. The standard electrode potential is the. the electrochemical series, also referred to as the activity series, is the ranking of metals organized by standard electrode potentials. importance and applications of electrochemical series. How Does The Electrochemical Series Work.
From classnotes.org.in
Electrochemical Series Chemistry, Class 12, Electro Chemistry How Does The Electrochemical Series Work this page explains the origin of the electrochemical series, and shows how it can be used to work out the ability of the. The standard electrode potential is the. the electrochemical series tells us how electropositive or electronegative the element/ion combination is, compared to standard hydrogen electrode. importance and applications of electrochemical series (with examples) 1) for. How Does The Electrochemical Series Work.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells How Does The Electrochemical Series Work the electrochemical series tells us how electropositive or electronegative the element/ion combination is, compared to standard hydrogen electrode. importance and applications of electrochemical series (with examples) 1) for comparison of the relative oxidizing. in the electrochemical series, metals and other substances are arranged in decreasing order of their tendency to gain. the electrochemical series, also referred. How Does The Electrochemical Series Work.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types How Does The Electrochemical Series Work the electrochemical series, also referred to as the activity series, is the ranking of metals organized by standard electrode potentials. importance and applications of electrochemical series (with examples) 1) for comparison of the relative oxidizing. the electrochemical series tells us how electropositive or electronegative the element/ion combination is, compared to standard hydrogen electrode. this page explains. How Does The Electrochemical Series Work.
From mavink.com
Reactivity Series Chart How Does The Electrochemical Series Work The standard electrode potential is the. in the electrochemical series, metals and other substances are arranged in decreasing order of their tendency to gain. the electrochemical series, also referred to as the activity series, is the ranking of metals organized by standard electrode potentials. this page explains the origin of the electrochemical series, and shows how it. How Does The Electrochemical Series Work.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells How Does The Electrochemical Series Work in the electrochemical series, metals and other substances are arranged in decreasing order of their tendency to gain. electrochemical series, also referred to as activity series, is a list that describes the arrangement of elements in the order of their increasing electrode potential. this page explains the origin of the electrochemical series, and shows how it can. How Does The Electrochemical Series Work.
From mungfali.com
What Is Electrochemical Series How Does The Electrochemical Series Work the electrochemical series tells us how electropositive or electronegative the element/ion combination is, compared to standard hydrogen electrode. the electrochemical series, also referred to as the activity series, is the ranking of metals organized by standard electrode potentials. importance and applications of electrochemical series (with examples) 1) for comparison of the relative oxidizing. in the electrochemical. How Does The Electrochemical Series Work.
From www.youtube.com
Electrochemical series (Electrochemistry part 16 for CBSE class 12 JEE IIT) YouTube How Does The Electrochemical Series Work in the electrochemical series, metals and other substances are arranged in decreasing order of their tendency to gain. the electrochemical series, also referred to as the activity series, is the ranking of metals organized by standard electrode potentials. the electrochemical series tells us how electropositive or electronegative the element/ion combination is, compared to standard hydrogen electrode. . How Does The Electrochemical Series Work.
From byjus.com
Electrochemical Series Definition, Char and Applications How Does The Electrochemical Series Work electrochemical series, also referred to as activity series, is a list that describes the arrangement of elements in the order of their increasing electrode potential. this page explains the origin of the electrochemical series, and shows how it can be used to work out the ability of the. The standard electrode potential is the. the electrochemical series,. How Does The Electrochemical Series Work.
From unacademy.com
Electrochemical Series, Features and Importance Unacademy How Does The Electrochemical Series Work this page explains the origin of the electrochemical series, and shows how it can be used to work out the ability of the. the electrochemical series, also referred to as the activity series, is the ranking of metals organized by standard electrode potentials. in the electrochemical series, metals and other substances are arranged in decreasing order of. How Does The Electrochemical Series Work.
From leverageedu.com
Electrochemical Series Notes Chemistry Class 11 & 12 Leverage Edu How Does The Electrochemical Series Work this page explains the origin of the electrochemical series, and shows how it can be used to work out the ability of the. the electrochemical series, also referred to as the activity series, is the ranking of metals organized by standard electrode potentials. importance and applications of electrochemical series (with examples) 1) for comparison of the relative. How Does The Electrochemical Series Work.
From mavink.com
Reactivity Series Chart How Does The Electrochemical Series Work electrochemical series, also referred to as activity series, is a list that describes the arrangement of elements in the order of their increasing electrode potential. this page explains the origin of the electrochemical series, and shows how it can be used to work out the ability of the. the electrochemical series, also referred to as the activity. How Does The Electrochemical Series Work.
From kanayatichemistry.blogspot.com
Reactivity series table K Chemistry How Does The Electrochemical Series Work the electrochemical series tells us how electropositive or electronegative the element/ion combination is, compared to standard hydrogen electrode. in the electrochemical series, metals and other substances are arranged in decreasing order of their tendency to gain. electrochemical series, also referred to as activity series, is a list that describes the arrangement of elements in the order of. How Does The Electrochemical Series Work.
From www.i2tutorials.com
Electrochemical Series i2tutorials How Does The Electrochemical Series Work electrochemical series, also referred to as activity series, is a list that describes the arrangement of elements in the order of their increasing electrode potential. the electrochemical series tells us how electropositive or electronegative the element/ion combination is, compared to standard hydrogen electrode. The standard electrode potential is the. the electrochemical series, also referred to as the. How Does The Electrochemical Series Work.
From thechemistrynotes.com
Electrochemical series and its applications How Does The Electrochemical Series Work in the electrochemical series, metals and other substances are arranged in decreasing order of their tendency to gain. importance and applications of electrochemical series (with examples) 1) for comparison of the relative oxidizing. electrochemical series, also referred to as activity series, is a list that describes the arrangement of elements in the order of their increasing electrode. How Does The Electrochemical Series Work.