Br2 2Br Exothermic . The opposite is true if we want to make new bonds. A chemical reaction or physical change is exothermic if heat is released by the system into the surroundings. Bond breaking is an endothermic process. An endothermic reaction is characterized by. Define endothermic and exothermic reactions. Determine if a chemical process is exothermic or endothermic. This is a good example of a. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. This can be used to classify reactions as exothermic or endothermic. Energy is released when new chemical bonds are. Use or interpret energy diagrams to. Because the surroundings are gaining heat. So to produce more bromine atoms (products),. The equilibrium constants for the reaction, br2 ⇌ 2br at 500k and 700k are 1×10−10 and 1×10−5 respectively.
from www.chegg.com
This can be used to classify reactions as exothermic or endothermic. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. Define endothermic and exothermic reactions. This is a good example of a. So to produce more bromine atoms (products),. Energy is released when new chemical bonds are. Bond breaking is an endothermic process. The equilibrium constants for the reaction, br2 ⇌ 2br at 500k and 700k are 1×10−10 and 1×10−5 respectively. Because the surroundings are gaining heat. Use or interpret energy diagrams to.
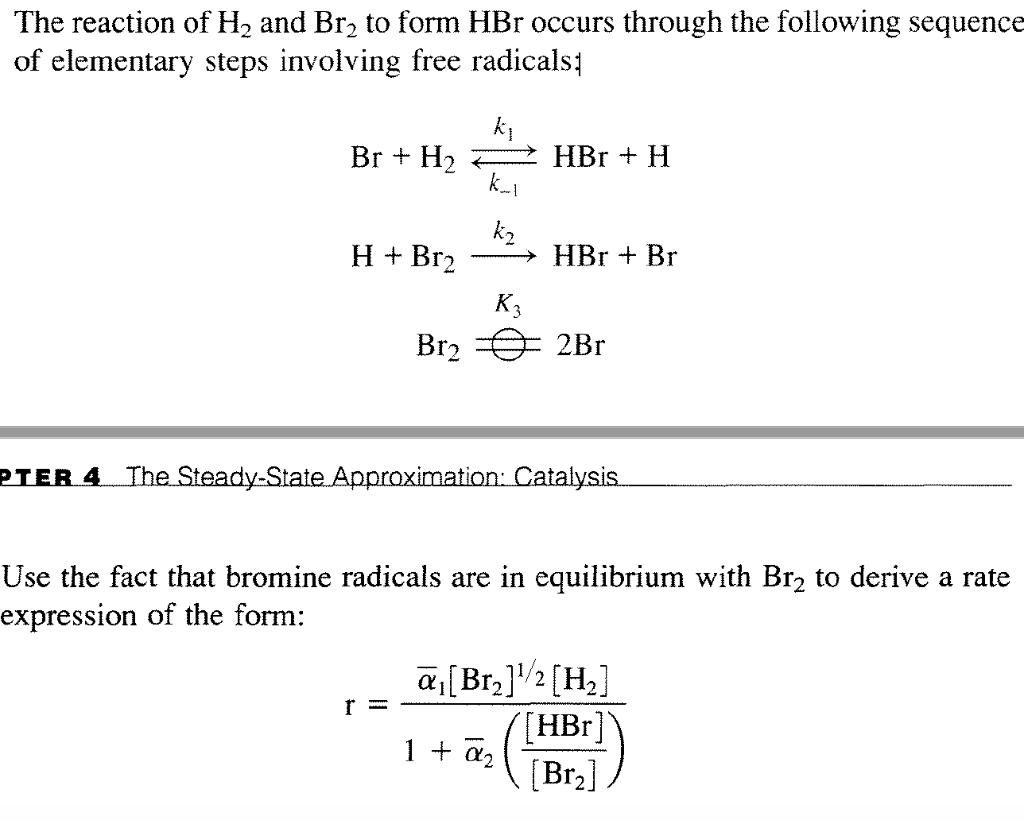
The reaction of H2 and Br2 to form HBr occurs through
Br2 2Br Exothermic Energy is released when new chemical bonds are. A chemical reaction or physical change is exothermic if heat is released by the system into the surroundings. The opposite is true if we want to make new bonds. This can be used to classify reactions as exothermic or endothermic. Determine if a chemical process is exothermic or endothermic. Bond breaking is an endothermic process. The equilibrium constants for the reaction, br2 ⇌ 2br at 500k and 700k are 1×10−10 and 1×10−5 respectively. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. An endothermic reaction is characterized by. Use or interpret energy diagrams to. Define endothermic and exothermic reactions. So to produce more bromine atoms (products),. This is a good example of a. Energy is released when new chemical bonds are. Because the surroundings are gaining heat.
From www.chegg.com
Solved H2(g) + Br2(g) → 2HBr(g) The following mechanism is Br2 2Br Exothermic An endothermic reaction is characterized by. Define endothermic and exothermic reactions. Determine if a chemical process is exothermic or endothermic. Energy is released when new chemical bonds are. A chemical reaction or physical change is exothermic if heat is released by the system into the surroundings. The equilibrium constants for the reaction, br2 ⇌ 2br at 500k and 700k are. Br2 2Br Exothermic.
From www.chegg.com
Solved Standard Reduction Potentials Br2() + 2e → 2Br(aq) Br2 2Br Exothermic The equilibrium constants for the reaction, br2 ⇌ 2br at 500k and 700k are 1×10−10 and 1×10−5 respectively. A chemical reaction or physical change is exothermic if heat is released by the system into the surroundings. An endothermic reaction is characterized by. The opposite is true if we want to make new bonds. This is a good example of a.. Br2 2Br Exothermic.
From www.slideserve.com
PPT Halogens PowerPoint Presentation, free download ID4396203 Br2 2Br Exothermic A chemical reaction or physical change is exothermic if heat is released by the system into the surroundings. This is a good example of a. Determine if a chemical process is exothermic or endothermic. The opposite is true if we want to make new bonds. Energy is released when new chemical bonds are. An endothermic reaction is characterized by. Because. Br2 2Br Exothermic.
From www.youtube.com
Br2 Lewis Structure How to Draw the Lewis Dot Structure for Dibromine Br2 2Br Exothermic Use or interpret energy diagrams to. Define endothermic and exothermic reactions. Because the surroundings are gaining heat. Determine if a chemical process is exothermic or endothermic. A chemical reaction or physical change is exothermic if heat is released by the system into the surroundings. The equilibrium constants for the reaction, br2 ⇌ 2br at 500k and 700k are 1×10−10 and. Br2 2Br Exothermic.
From www.numerade.com
SOLVED For the following reaction, select all the statements that are Br2 2Br Exothermic The opposite is true if we want to make new bonds. Energy is released when new chemical bonds are. Bond breaking is an endothermic process. Use or interpret energy diagrams to. The equilibrium constants for the reaction, br2 ⇌ 2br at 500k and 700k are 1×10−10 and 1×10−5 respectively. Because the surroundings are gaining heat. Define endothermic and exothermic reactions.. Br2 2Br Exothermic.
From www.numerade.com
SOLVED A voltaic electrochemical cell is constructed in which the Br2 2Br Exothermic Because the surroundings are gaining heat. So to produce more bromine atoms (products),. Energy is released when new chemical bonds are. Bond breaking is an endothermic process. Use or interpret energy diagrams to. This is a good example of a. The opposite is true if we want to make new bonds. The changes in energy that occur during a chemical. Br2 2Br Exothermic.
From www.toppr.com
The stoichiometric equation for the oxidation of bromide ions by Br2 2Br Exothermic Bond breaking is an endothermic process. Define endothermic and exothermic reactions. So to produce more bromine atoms (products),. This can be used to classify reactions as exothermic or endothermic. This is a good example of a. Energy is released when new chemical bonds are. Determine if a chemical process is exothermic or endothermic. The opposite is true if we want. Br2 2Br Exothermic.
From manualfixdercombustion.z13.web.core.windows.net
Br2 Molecular Orbital Diagram Br2 2Br Exothermic This is a good example of a. Bond breaking is an endothermic process. The opposite is true if we want to make new bonds. Because the surroundings are gaining heat. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. An endothermic reaction is characterized by. Use or interpret energy. Br2 2Br Exothermic.
From www.numerade.com
SOLVED Balance the following equation under both acidic and basic Br2 2Br Exothermic The equilibrium constants for the reaction, br2 ⇌ 2br at 500k and 700k are 1×10−10 and 1×10−5 respectively. Energy is released when new chemical bonds are. The opposite is true if we want to make new bonds. Define endothermic and exothermic reactions. So to produce more bromine atoms (products),. The changes in energy that occur during a chemical reaction can. Br2 2Br Exothermic.
From www.youtube.com
How to find the Oxidation Number for Br in Br2 (Bromine gas) YouTube Br2 2Br Exothermic The opposite is true if we want to make new bonds. This is a good example of a. Define endothermic and exothermic reactions. A chemical reaction or physical change is exothermic if heat is released by the system into the surroundings. The equilibrium constants for the reaction, br2 ⇌ 2br at 500k and 700k are 1×10−10 and 1×10−5 respectively. This. Br2 2Br Exothermic.
From www.numerade.com
A weighed sample of iron (Fe) is added to liquid bromine (Br2) and Br2 2Br Exothermic Because the surroundings are gaining heat. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. Use or interpret energy diagrams to. So to produce more bromine atoms (products),. Bond breaking is an endothermic process. The equilibrium constants for the reaction, br2 ⇌ 2br at 500k and 700k are 1×10−10. Br2 2Br Exothermic.
From www.chegg.com
Solved MCQ Which reaction is rarer to occur * Br2→2Br Br2 2Br Exothermic Determine if a chemical process is exothermic or endothermic. Energy is released when new chemical bonds are. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. This can be used to classify reactions as exothermic or endothermic. So to produce more bromine atoms (products),. An endothermic reaction is characterized. Br2 2Br Exothermic.
From www.chegg.com
Solved Consider the following mechanism Step 1Br2→2Br Step Br2 2Br Exothermic A chemical reaction or physical change is exothermic if heat is released by the system into the surroundings. The equilibrium constants for the reaction, br2 ⇌ 2br at 500k and 700k are 1×10−10 and 1×10−5 respectively. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. So to produce more. Br2 2Br Exothermic.
From www.chegg.com
Solved 1. Cl2 (aq) + 2Br (ag) Br2+2Cl (ag) 2. Cl2 (ag) + Br2 2Br Exothermic Use or interpret energy diagrams to. Energy is released when new chemical bonds are. The equilibrium constants for the reaction, br2 ⇌ 2br at 500k and 700k are 1×10−10 and 1×10−5 respectively. Bond breaking is an endothermic process. This is a good example of a. The changes in energy that occur during a chemical reaction can be seen by examining. Br2 2Br Exothermic.
From askfilo.com
The equilibrium constant for the reaction Br2 ⇌ 2Br at 500 K and 700 K ar.. Br2 2Br Exothermic This can be used to classify reactions as exothermic or endothermic. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. An endothermic reaction is characterized by. Energy is released when new chemical bonds are. This is a good example of a. Because the surroundings are gaining heat. A chemical. Br2 2Br Exothermic.
From www.numerade.com
SOLVED For the reaction 2HBr → H2 + Br2 ΔH = 17.4 kcal/mol. And Br2 2Br Exothermic Use or interpret energy diagrams to. Determine if a chemical process is exothermic or endothermic. Bond breaking is an endothermic process. An endothermic reaction is characterized by. The opposite is true if we want to make new bonds. This is a good example of a. The equilibrium constants for the reaction, br2 ⇌ 2br at 500k and 700k are 1×10−10. Br2 2Br Exothermic.
From slideplayer.com
Acids and Bases L. ppt download Br2 2Br Exothermic Define endothermic and exothermic reactions. This is a good example of a. Energy is released when new chemical bonds are. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. A chemical reaction or physical change is exothermic if heat is released by the system into the surroundings. An endothermic. Br2 2Br Exothermic.
From www.numerade.com
SOLVED Classify each reaction as endothermic or exothermic. H2 + Br2 → Br2 2Br Exothermic An endothermic reaction is characterized by. Because the surroundings are gaining heat. The opposite is true if we want to make new bonds. This can be used to classify reactions as exothermic or endothermic. The equilibrium constants for the reaction, br2 ⇌ 2br at 500k and 700k are 1×10−10 and 1×10−5 respectively. Bond breaking is an endothermic process. Determine if. Br2 2Br Exothermic.
From www.toppr.com
The rate law of the reaction H2 + Br2→ 2HBr is d[HBr]dt = k[H2][Br2]^1 Br2 2Br Exothermic The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. Use or interpret energy diagrams to. This is a good example of a. The equilibrium constants for the reaction, br2 ⇌ 2br at 500k and 700k are 1×10−10 and 1×10−5 respectively. Because the surroundings are gaining heat. Determine if a. Br2 2Br Exothermic.
From www.toppr.com
Consider the reaction, Cl2(g) + 2Br^(aq.) → 2Cl^(aq.) + Br2. The emf Br2 2Br Exothermic The opposite is true if we want to make new bonds. Energy is released when new chemical bonds are. A chemical reaction or physical change is exothermic if heat is released by the system into the surroundings. Because the surroundings are gaining heat. Define endothermic and exothermic reactions. Use or interpret energy diagrams to. An endothermic reaction is characterized by.. Br2 2Br Exothermic.
From www.toppr.com
Consider the reaction, Cl2(g) + 2Br^(aq.) → 2Cl^(aq.) + Br2. The emf Br2 2Br Exothermic The opposite is true if we want to make new bonds. A chemical reaction or physical change is exothermic if heat is released by the system into the surroundings. An endothermic reaction is characterized by. This can be used to classify reactions as exothermic or endothermic. So to produce more bromine atoms (products),. Bond breaking is an endothermic process. Define. Br2 2Br Exothermic.
From www.chegg.com
Solved 2CI+ Br2 2Br + Cl2 In the above reaction, the Br2 2Br Exothermic An endothermic reaction is characterized by. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. This can be used to classify reactions as exothermic or endothermic. So to produce more bromine atoms (products),. A chemical reaction or physical change is exothermic if heat is released by the system into. Br2 2Br Exothermic.
From www.chegg.com
4. The detailed reaction mechanism for the reaction Br2 2Br Exothermic A chemical reaction or physical change is exothermic if heat is released by the system into the surroundings. So to produce more bromine atoms (products),. Because the surroundings are gaining heat. Use or interpret energy diagrams to. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. Energy is released. Br2 2Br Exothermic.
From www.youtube.com
Type of Reaction for KI + Br2 = KBr + I2 YouTube Br2 2Br Exothermic So to produce more bromine atoms (products),. Bond breaking is an endothermic process. This can be used to classify reactions as exothermic or endothermic. Use or interpret energy diagrams to. Define endothermic and exothermic reactions. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. The opposite is true if. Br2 2Br Exothermic.
From www.bartleby.com
Answered Consider the following halfreactions… bartleby Br2 2Br Exothermic An endothermic reaction is characterized by. The opposite is true if we want to make new bonds. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. Define endothermic and exothermic reactions. Determine if a chemical process is exothermic or endothermic. This is a good example of a. A chemical. Br2 2Br Exothermic.
From www.chegg.com
Solved Question 10 of 10 If the reaction H2 + Br2 → 2HBr has Br2 2Br Exothermic Use or interpret energy diagrams to. Bond breaking is an endothermic process. So to produce more bromine atoms (products),. Define endothermic and exothermic reactions. Energy is released when new chemical bonds are. Because the surroundings are gaining heat. The equilibrium constants for the reaction, br2 ⇌ 2br at 500k and 700k are 1×10−10 and 1×10−5 respectively. Determine if a chemical. Br2 2Br Exothermic.
From www.numerade.com
SOLVEDThe following reaction is exothermic. C2 H4(g)+Br2(g) ⇌C2 H4 Br2 Br2 2Br Exothermic Determine if a chemical process is exothermic or endothermic. Define endothermic and exothermic reactions. This can be used to classify reactions as exothermic or endothermic. The opposite is true if we want to make new bonds. Because the surroundings are gaining heat. A chemical reaction or physical change is exothermic if heat is released by the system into the surroundings.. Br2 2Br Exothermic.
From www.toppr.com
Consider the reaction; Cl2(g) + 2Br^ (aq)→ 2Cl^ (aq) + Br2 The emf Br2 2Br Exothermic The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. So to produce more bromine atoms (products),. Determine if a chemical process is exothermic or endothermic. Use or interpret energy diagrams to. Because the surroundings are gaining heat. Energy is released when new chemical bonds are. An endothermic reaction is. Br2 2Br Exothermic.
From www.gauthmath.com
Solved Balance this half equation by identifying the number of Br2 2Br Exothermic Determine if a chemical process is exothermic or endothermic. This can be used to classify reactions as exothermic or endothermic. So to produce more bromine atoms (products),. This is a good example of a. An endothermic reaction is characterized by. Use or interpret energy diagrams to. Define endothermic and exothermic reactions. Bond breaking is an endothermic process. The changes in. Br2 2Br Exothermic.
From www.numerade.com
SOLVED Be sure to answer all parts. Use standard reduction potentials Br2 2Br Exothermic Because the surroundings are gaining heat. A chemical reaction or physical change is exothermic if heat is released by the system into the surroundings. So to produce more bromine atoms (products),. Bond breaking is an endothermic process. Determine if a chemical process is exothermic or endothermic. Use or interpret energy diagrams to. The equilibrium constants for the reaction, br2 ⇌. Br2 2Br Exothermic.
From www.slideserve.com
PPT The 1 st Law of Thermodynamics The energy of the universe is Br2 2Br Exothermic Use or interpret energy diagrams to. Bond breaking is an endothermic process. Energy is released when new chemical bonds are. The opposite is true if we want to make new bonds. This can be used to classify reactions as exothermic or endothermic. Define endothermic and exothermic reactions. The changes in energy that occur during a chemical reaction can be seen. Br2 2Br Exothermic.
From www.numerade.com
SOLVED Is the dissociation of bromine molecules into atomic bromine Br2 2Br Exothermic Because the surroundings are gaining heat. Define endothermic and exothermic reactions. Use or interpret energy diagrams to. Bond breaking is an endothermic process. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. This is a good example of a. An endothermic reaction is characterized by. So to produce more. Br2 2Br Exothermic.
From www.toppr.com
The equilibrium constant for the reaction Br2 2Br at 500 K and 700 K Br2 2Br Exothermic Bond breaking is an endothermic process. So to produce more bromine atoms (products),. Define endothermic and exothermic reactions. Use or interpret energy diagrams to. Energy is released when new chemical bonds are. Because the surroundings are gaining heat. This can be used to classify reactions as exothermic or endothermic. The equilibrium constants for the reaction, br2 ⇌ 2br at 500k. Br2 2Br Exothermic.
From www.chegg.com
The reaction of H2 and Br2 to form HBr occurs through Br2 2Br Exothermic An endothermic reaction is characterized by. Bond breaking is an endothermic process. This can be used to classify reactions as exothermic or endothermic. Define endothermic and exothermic reactions. Energy is released when new chemical bonds are. Determine if a chemical process is exothermic or endothermic. The equilibrium constants for the reaction, br2 ⇌ 2br at 500k and 700k are 1×10−10. Br2 2Br Exothermic.
From www.numerade.com
Cl2 (aq) + 2Br (aq) > Br2 (aq) + 2Cl (aq) a. Is this reaction an Br2 2Br Exothermic The equilibrium constants for the reaction, br2 ⇌ 2br at 500k and 700k are 1×10−10 and 1×10−5 respectively. This is a good example of a. Bond breaking is an endothermic process. Because the surroundings are gaining heat. Use or interpret energy diagrams to. A chemical reaction or physical change is exothermic if heat is released by the system into the. Br2 2Br Exothermic.