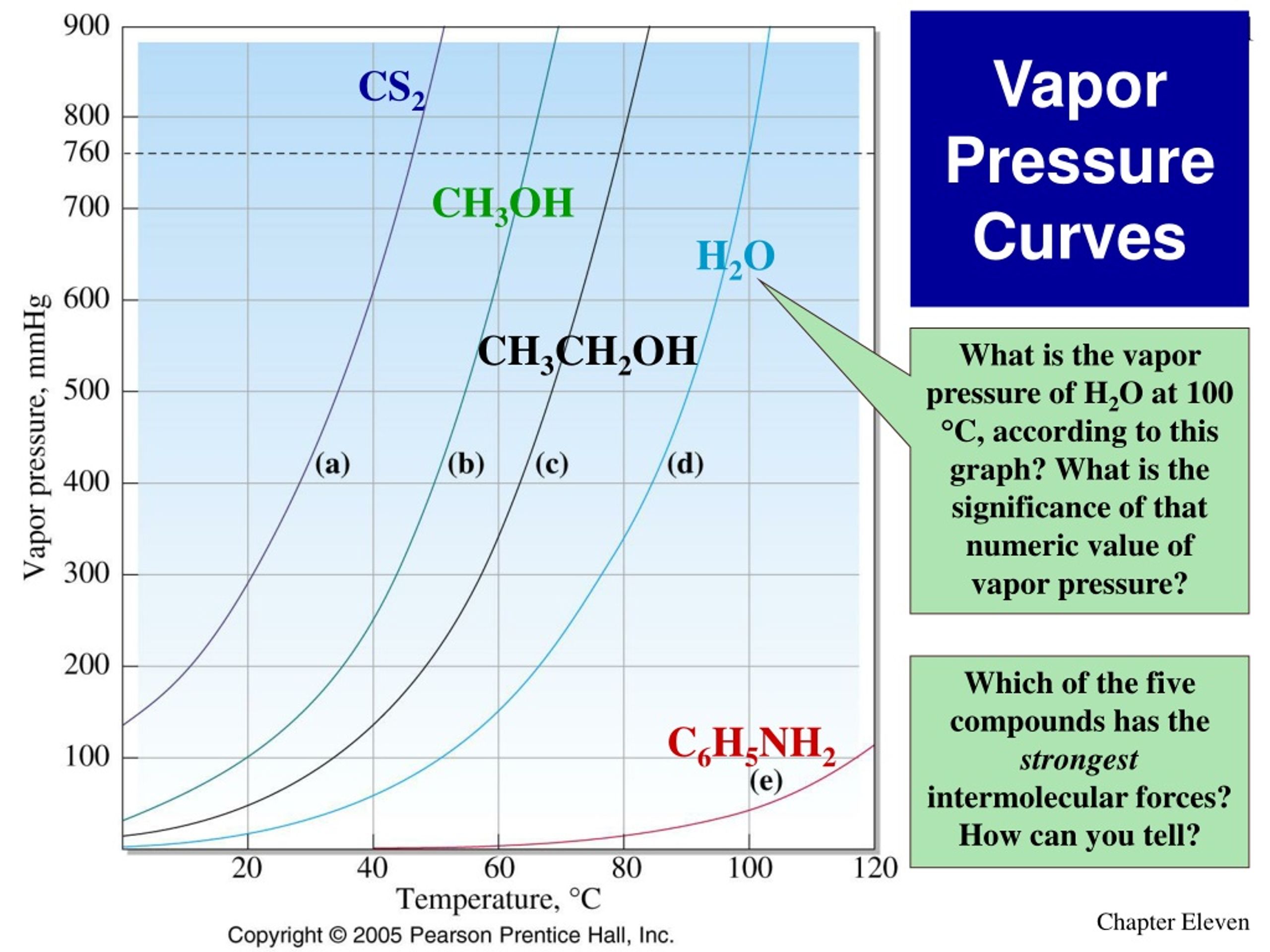
Does Vapor Pressure Increase With Temperature . See examples, graphs and exercises on vapor pressure and its relationship with. Learn how vapor pressure is defined and how it depends on intermolecular forces and temperature. The vapor pressure of a liquid increases with increasing temperature. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open container. Vapor pressure is the equilibrium pressure of a vapor above a liquid or solid in a closed container. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Substances with weaker intermolecular forces will have a higher vapor pressure at any. Learn how vapor pressure varies with temperature, types of molecules and surface area, and see. Increasing the temperature will increase the vapor pressure. If the heat of vaporization and the vapor pressure at one temperature are.
from www.slideserve.com
Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. The vapor pressure of a liquid increases with increasing temperature. If the heat of vaporization and the vapor pressure at one temperature are. Learn how vapor pressure is defined and how it depends on intermolecular forces and temperature. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open container. See examples, graphs and exercises on vapor pressure and its relationship with. Learn how vapor pressure varies with temperature, types of molecules and surface area, and see. Increasing the temperature will increase the vapor pressure. Substances with weaker intermolecular forces will have a higher vapor pressure at any. Vapor pressure is the equilibrium pressure of a vapor above a liquid or solid in a closed container.
PPT States of Matter and Intermolecular Forces PowerPoint
Does Vapor Pressure Increase With Temperature Learn how vapor pressure is defined and how it depends on intermolecular forces and temperature. If the heat of vaporization and the vapor pressure at one temperature are. Increasing the temperature will increase the vapor pressure. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open container. Vapor pressure is the equilibrium pressure of a vapor above a liquid or solid in a closed container. The vapor pressure of a liquid increases with increasing temperature. Learn how vapor pressure varies with temperature, types of molecules and surface area, and see. Substances with weaker intermolecular forces will have a higher vapor pressure at any. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Learn how vapor pressure is defined and how it depends on intermolecular forces and temperature. See examples, graphs and exercises on vapor pressure and its relationship with.
From slideplayer.com
Temperature and Pressure ppt download Does Vapor Pressure Increase With Temperature Learn how vapor pressure is defined and how it depends on intermolecular forces and temperature. Increasing the temperature will increase the vapor pressure. See examples, graphs and exercises on vapor pressure and its relationship with. Learn how vapor pressure varies with temperature, types of molecules and surface area, and see. If the heat of vaporization and the vapor pressure at. Does Vapor Pressure Increase With Temperature.
From slideplayer.com
Aim How does temperature affect the vapor pressure of liquids Does Vapor Pressure Increase With Temperature If the heat of vaporization and the vapor pressure at one temperature are. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open container. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Learn. Does Vapor Pressure Increase With Temperature.
From www.slideserve.com
PPT States of Matter and Intermolecular Forces PowerPoint Does Vapor Pressure Increase With Temperature If the heat of vaporization and the vapor pressure at one temperature are. Learn how vapor pressure varies with temperature, types of molecules and surface area, and see. See examples, graphs and exercises on vapor pressure and its relationship with. Learn how vapor pressure is defined and how it depends on intermolecular forces and temperature. The vapor pressure of a. Does Vapor Pressure Increase With Temperature.
From engineerexcel.com
Pressure Temperature Graphs Explained EngineerExcel Does Vapor Pressure Increase With Temperature Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. See examples, graphs and exercises on vapor pressure and its relationship with. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open container. Learn how. Does Vapor Pressure Increase With Temperature.
From socratic.org
Explain how each of the following affects the vapour pressure of a Does Vapor Pressure Increase With Temperature The vapor pressure of a liquid increases with increasing temperature. If the heat of vaporization and the vapor pressure at one temperature are. Increasing the temperature will increase the vapor pressure. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an. Does Vapor Pressure Increase With Temperature.
From slideplayer.com
Liquids, Solids, and Intermolecular Forces ppt download Does Vapor Pressure Increase With Temperature Substances with weaker intermolecular forces will have a higher vapor pressure at any. See examples, graphs and exercises on vapor pressure and its relationship with. Vapor pressure is the equilibrium pressure of a vapor above a liquid or solid in a closed container. Learn how vapor pressure is defined and how it depends on intermolecular forces and temperature. The vapor. Does Vapor Pressure Increase With Temperature.
From www.slideserve.com
PPT Why . . . PowerPoint Presentation, free download ID2250075 Does Vapor Pressure Increase With Temperature Learn how vapor pressure is defined and how it depends on intermolecular forces and temperature. Increasing the temperature will increase the vapor pressure. See examples, graphs and exercises on vapor pressure and its relationship with. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Learn how vapor pressure varies with temperature, types of molecules. Does Vapor Pressure Increase With Temperature.
From slideplayer.com
Aim How does temperature affect the vapor pressure of liquids Does Vapor Pressure Increase With Temperature See examples, graphs and exercises on vapor pressure and its relationship with. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open container. Increasing the temperature will increase the vapor pressure. Learn how vapor pressure is defined and how it. Does Vapor Pressure Increase With Temperature.
From slideplayer.com
Aim How does temperature affect the vapor pressure of liquids Does Vapor Pressure Increase With Temperature If the heat of vaporization and the vapor pressure at one temperature are. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open container. Substances with weaker intermolecular forces will have a higher vapor pressure at any. Generally a substance's. Does Vapor Pressure Increase With Temperature.
From shaunmwilliams.com
Chapter 10 Presentation Does Vapor Pressure Increase With Temperature Increasing the temperature will increase the vapor pressure. Learn how vapor pressure varies with temperature, types of molecules and surface area, and see. Substances with weaker intermolecular forces will have a higher vapor pressure at any. The vapor pressure of a liquid increases with increasing temperature. Learn how vapor pressure is defined and how it depends on intermolecular forces and. Does Vapor Pressure Increase With Temperature.
From slideplayer.com
Aim How does temperature affect the vapor pressure of liquids Does Vapor Pressure Increase With Temperature As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open container. Learn how vapor pressure varies with temperature, types of molecules and surface area, and see. Increasing the temperature will increase the vapor pressure. Vapor pressure is the equilibrium pressure. Does Vapor Pressure Increase With Temperature.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID5080574 Does Vapor Pressure Increase With Temperature See examples, graphs and exercises on vapor pressure and its relationship with. If the heat of vaporization and the vapor pressure at one temperature are. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open container. Learn how vapor pressure. Does Vapor Pressure Increase With Temperature.
From loebantdv.blob.core.windows.net
Why Does Equilibrium Vapor Pressure Increase With Temperature at Thomas Does Vapor Pressure Increase With Temperature Vapor pressure is the equilibrium pressure of a vapor above a liquid or solid in a closed container. See examples, graphs and exercises on vapor pressure and its relationship with. Substances with weaker intermolecular forces will have a higher vapor pressure at any. Learn how vapor pressure varies with temperature, types of molecules and surface area, and see. As the. Does Vapor Pressure Increase With Temperature.
From ecampusontario.pressbooks.pub
Appendix F Water Properties General Chemistry for GeeGees Does Vapor Pressure Increase With Temperature Vapor pressure is the equilibrium pressure of a vapor above a liquid or solid in a closed container. See examples, graphs and exercises on vapor pressure and its relationship with. Substances with weaker intermolecular forces will have a higher vapor pressure at any. Learn how vapor pressure is defined and how it depends on intermolecular forces and temperature. If the. Does Vapor Pressure Increase With Temperature.
From www.slideserve.com
PPT Why . . . PowerPoint Presentation, free download ID2250075 Does Vapor Pressure Increase With Temperature As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open container. Vapor pressure is the equilibrium pressure of a vapor above a liquid or solid in a closed container. If the heat of vaporization and the vapor pressure at one. Does Vapor Pressure Increase With Temperature.
From www.slideserve.com
PPT States of Matter and Intermolecular Forces PowerPoint Does Vapor Pressure Increase With Temperature Increasing the temperature will increase the vapor pressure. See examples, graphs and exercises on vapor pressure and its relationship with. Learn how vapor pressure varies with temperature, types of molecules and surface area, and see. If the heat of vaporization and the vapor pressure at one temperature are. As the temperature of a liquid increases, the vapor pressure of the. Does Vapor Pressure Increase With Temperature.
From www.researchgate.net
The effect of temperature on saturation vapor pressure, actual vapor Does Vapor Pressure Increase With Temperature As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open container. Vapor pressure is the equilibrium pressure of a vapor above a liquid or solid in a closed container. Learn how vapor pressure is defined and how it depends on. Does Vapor Pressure Increase With Temperature.
From slideplayer.com
Aim How does temperature affect the vapor pressure of liquids Does Vapor Pressure Increase With Temperature If the heat of vaporization and the vapor pressure at one temperature are. Increasing the temperature will increase the vapor pressure. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. See examples, graphs and exercises on vapor pressure and its relationship with. The vapor pressure of a liquid increases with increasing temperature. Learn how. Does Vapor Pressure Increase With Temperature.
From loebantdv.blob.core.windows.net
Why Does Equilibrium Vapor Pressure Increase With Temperature at Thomas Does Vapor Pressure Increase With Temperature See examples, graphs and exercises on vapor pressure and its relationship with. Learn how vapor pressure is defined and how it depends on intermolecular forces and temperature. Substances with weaker intermolecular forces will have a higher vapor pressure at any. If the heat of vaporization and the vapor pressure at one temperature are. Increasing the temperature will increase the vapor. Does Vapor Pressure Increase With Temperature.
From www.chemistrylibrary.org
Vapor Pressure Does Vapor Pressure Increase With Temperature If the heat of vaporization and the vapor pressure at one temperature are. Increasing the temperature will increase the vapor pressure. Vapor pressure is the equilibrium pressure of a vapor above a liquid or solid in a closed container. Substances with weaker intermolecular forces will have a higher vapor pressure at any. As the temperature of a liquid increases, the. Does Vapor Pressure Increase With Temperature.
From techblog.ctgclean.com
Drying Vapor Pressure vs. Temperature CTG Clean Does Vapor Pressure Increase With Temperature The vapor pressure of a liquid increases with increasing temperature. Substances with weaker intermolecular forces will have a higher vapor pressure at any. If the heat of vaporization and the vapor pressure at one temperature are. Vapor pressure is the equilibrium pressure of a vapor above a liquid or solid in a closed container. Generally a substance's vapor pressure increases. Does Vapor Pressure Increase With Temperature.
From sciencenotes.org
Vapor Pressure Definition and How to Calculate It Does Vapor Pressure Increase With Temperature The vapor pressure of a liquid increases with increasing temperature. Increasing the temperature will increase the vapor pressure. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. If the heat of vaporization and the vapor pressure at one temperature are. As the temperature of a liquid increases, the vapor pressure of the liquid increases. Does Vapor Pressure Increase With Temperature.
From www.slideserve.com
PPT Chapter 11 Liquids & Solids PowerPoint Presentation, free Does Vapor Pressure Increase With Temperature If the heat of vaporization and the vapor pressure at one temperature are. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. See examples, graphs and exercises on vapor pressure and its relationship with. Learn how vapor pressure varies with temperature, types of molecules and surface area, and see. Increasing the temperature will increase. Does Vapor Pressure Increase With Temperature.
From jmpcoblog.com
Avoiding Pump Cavitation in Open Systems The Dynamic Relationship Does Vapor Pressure Increase With Temperature Substances with weaker intermolecular forces will have a higher vapor pressure at any. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open container. Learn. Does Vapor Pressure Increase With Temperature.
From www.slideserve.com
PPT Liquids and Solids PowerPoint Presentation, free download ID765930 Does Vapor Pressure Increase With Temperature See examples, graphs and exercises on vapor pressure and its relationship with. Vapor pressure is the equilibrium pressure of a vapor above a liquid or solid in a closed container. Increasing the temperature will increase the vapor pressure. If the heat of vaporization and the vapor pressure at one temperature are. Learn how vapor pressure varies with temperature, types of. Does Vapor Pressure Increase With Temperature.
From www.numerade.com
SOLVED does vapor pressure increase or decrease with temperature Does Vapor Pressure Increase With Temperature If the heat of vaporization and the vapor pressure at one temperature are. Increasing the temperature will increase the vapor pressure. Learn how vapor pressure is defined and how it depends on intermolecular forces and temperature. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure. Does Vapor Pressure Increase With Temperature.
From mungfali.com
Water Vapor Pressure Temperature Chart Does Vapor Pressure Increase With Temperature Learn how vapor pressure varies with temperature, types of molecules and surface area, and see. Substances with weaker intermolecular forces will have a higher vapor pressure at any. Increasing the temperature will increase the vapor pressure. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Vapor pressure is the equilibrium pressure of a vapor. Does Vapor Pressure Increase With Temperature.
From www.slideserve.com
PPT Evaporation, Vapor Pressure, and Intermolecular Forces PowerPoint Does Vapor Pressure Increase With Temperature The vapor pressure of a liquid increases with increasing temperature. If the heat of vaporization and the vapor pressure at one temperature are. Learn how vapor pressure varies with temperature, types of molecules and surface area, and see. Increasing the temperature will increase the vapor pressure. Vapor pressure is the equilibrium pressure of a vapor above a liquid or solid. Does Vapor Pressure Increase With Temperature.
From www.slideserve.com
PPT The Ideal Gas Equation PowerPoint Presentation, free download Does Vapor Pressure Increase With Temperature The vapor pressure of a liquid increases with increasing temperature. Substances with weaker intermolecular forces will have a higher vapor pressure at any. Learn how vapor pressure varies with temperature, types of molecules and surface area, and see. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Vapor pressure is the equilibrium pressure of. Does Vapor Pressure Increase With Temperature.
From www.researchgate.net
Vapor pressure and density of water vapor as function of temperature Does Vapor Pressure Increase With Temperature The vapor pressure of a liquid increases with increasing temperature. If the heat of vaporization and the vapor pressure at one temperature are. Learn how vapor pressure is defined and how it depends on intermolecular forces and temperature. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Vapor pressure is the equilibrium pressure of. Does Vapor Pressure Increase With Temperature.
From slideplayer.com
ATMOSPHERIC MOISTURE (Chapter 4). ppt download Does Vapor Pressure Increase With Temperature As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open container. The vapor pressure of a liquid increases with increasing temperature. Vapor pressure is the equilibrium pressure of a vapor above a liquid or solid in a closed container. Generally. Does Vapor Pressure Increase With Temperature.
From www.slideserve.com
PPT Ch. 11 Liquids, Solids, and Intermolecular Forces PowerPoint Does Vapor Pressure Increase With Temperature Substances with weaker intermolecular forces will have a higher vapor pressure at any. Increasing the temperature will increase the vapor pressure. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open container. Vapor pressure is the equilibrium pressure of a. Does Vapor Pressure Increase With Temperature.
From classnotes.org.in
Vapour Pressure Chemistry, Class 11, States of Matter Does Vapor Pressure Increase With Temperature Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Learn how vapor pressure is defined and how it depends on intermolecular forces and temperature. The vapor pressure of a liquid increases with increasing temperature. Learn how vapor pressure varies with temperature, types of molecules and surface area, and see. See examples, graphs and exercises. Does Vapor Pressure Increase With Temperature.
From www.researchgate.net
Vapour pressure versus saturation temperature. Download Scientific Does Vapor Pressure Increase With Temperature Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. If the heat of vaporization and the vapor pressure at one temperature are. See examples, graphs and exercises on vapor pressure and its relationship with. Learn how vapor pressure varies with temperature, types of molecules and surface area, and see. Increasing the temperature will increase. Does Vapor Pressure Increase With Temperature.
From www.slideserve.com
PPT 17 Equilibrium (AHL) PowerPoint Presentation, free download ID Does Vapor Pressure Increase With Temperature Substances with weaker intermolecular forces will have a higher vapor pressure at any. If the heat of vaporization and the vapor pressure at one temperature are. Vapor pressure is the equilibrium pressure of a vapor above a liquid or solid in a closed container. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. The. Does Vapor Pressure Increase With Temperature.