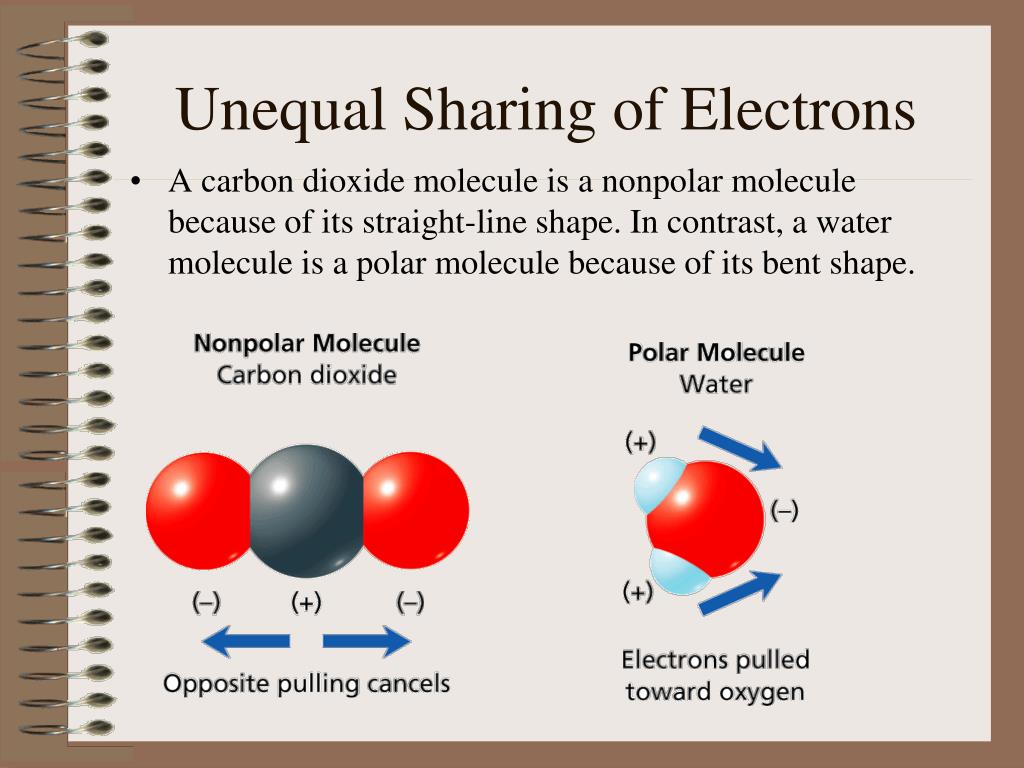
What Is A Result Of The Unequal Electron Sharing In A Water Molecule . The unequal sharing of electrons gives the water molecule a slight negative charge near its oxygen atom and a slight positive charge near its. It makes it a polar molecule. What is the result of these covalent. Are the electrons in a water molecule shared equally? Because of the higher electronegativity of the oxygen atom, the bonds are polar. Unequal distribution of charge in a molecule. The unequal sharing of electrons between the atoms and the unsymmetrical shape of the molecule means that a water molecule has two. In water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. What does the unequal sharing of electrons within a water molecule make the water molecule? Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. A polar covalent bond is a covalent bond in which the atoms have an unequal attraction for electrons and so the sharing is.
from www.slideserve.com
The unequal sharing of electrons gives the water molecule a slight negative charge near its oxygen atom and a slight positive charge near its. What is the result of these covalent. Unequal distribution of charge in a molecule. It makes it a polar molecule. Because of the higher electronegativity of the oxygen atom, the bonds are polar. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. The unequal sharing of electrons between the atoms and the unsymmetrical shape of the molecule means that a water molecule has two. What does the unequal sharing of electrons within a water molecule make the water molecule? In water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. Are the electrons in a water molecule shared equally?
PPT Notes 53 Covalent Bonds PowerPoint Presentation, free download
What Is A Result Of The Unequal Electron Sharing In A Water Molecule In water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. What is the result of these covalent. A polar covalent bond is a covalent bond in which the atoms have an unequal attraction for electrons and so the sharing is. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. What does the unequal sharing of electrons within a water molecule make the water molecule? It makes it a polar molecule. Unequal distribution of charge in a molecule. The unequal sharing of electrons between the atoms and the unsymmetrical shape of the molecule means that a water molecule has two. The unequal sharing of electrons gives the water molecule a slight negative charge near its oxygen atom and a slight positive charge near its. Are the electrons in a water molecule shared equally? In water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. Because of the higher electronegativity of the oxygen atom, the bonds are polar.
From biolo1100.nicerweb.com
bondpolar/02_11_01 What Is A Result Of The Unequal Electron Sharing In A Water Molecule It makes it a polar molecule. The unequal sharing of electrons gives the water molecule a slight negative charge near its oxygen atom and a slight positive charge near its. In water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. A polar covalent bond is a covalent. What Is A Result Of The Unequal Electron Sharing In A Water Molecule.
From www.numerade.com
SOLVED 1point water molecule, as shown here, IS polar because of H H What Is A Result Of The Unequal Electron Sharing In A Water Molecule What does the unequal sharing of electrons within a water molecule make the water molecule? Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Unequal distribution of charge in a molecule. The unequal sharing of electrons between the atoms and the unsymmetrical shape of the molecule means that a water molecule has two.. What Is A Result Of The Unequal Electron Sharing In A Water Molecule.
From www.numerade.com
SOLVED Page 4 33. Why do the atoms of a water molecule have partial What Is A Result Of The Unequal Electron Sharing In A Water Molecule Are the electrons in a water molecule shared equally? Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Unequal distribution of charge in a molecule. The unequal sharing of electrons gives the water molecule a slight negative charge near its oxygen atom and a slight positive charge near its. What does the unequal. What Is A Result Of The Unequal Electron Sharing In A Water Molecule.
From slideplayer.com
Copyright Pearson Prentice Hall ppt download What Is A Result Of The Unequal Electron Sharing In A Water Molecule What does the unequal sharing of electrons within a water molecule make the water molecule? The unequal sharing of electrons between the atoms and the unsymmetrical shape of the molecule means that a water molecule has two. Because of the higher electronegativity of the oxygen atom, the bonds are polar. Are the electrons in a water molecule shared equally? Unequal. What Is A Result Of The Unequal Electron Sharing In A Water Molecule.
From www.slideserve.com
PPT Notes 53 Covalent Bonds PowerPoint Presentation, free download What Is A Result Of The Unequal Electron Sharing In A Water Molecule The unequal sharing of electrons between the atoms and the unsymmetrical shape of the molecule means that a water molecule has two. What is the result of these covalent. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. What does the unequal sharing of electrons within a water molecule make the water molecule?. What Is A Result Of The Unequal Electron Sharing In A Water Molecule.
From slideplayer.com
Polar covalent bond unequal sharing of electrons ppt download What Is A Result Of The Unequal Electron Sharing In A Water Molecule What does the unequal sharing of electrons within a water molecule make the water molecule? Unequal distribution of charge in a molecule. The unequal sharing of electrons between the atoms and the unsymmetrical shape of the molecule means that a water molecule has two. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms.. What Is A Result Of The Unequal Electron Sharing In A Water Molecule.
From slideplayer.com
A. Definitions Solution homogeneous mixture ppt download What Is A Result Of The Unequal Electron Sharing In A Water Molecule What is the result of these covalent. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Unequal distribution of charge in a molecule. The unequal sharing of electrons gives the water molecule a slight negative charge near its oxygen atom and a slight positive charge near its. In water, each hydrogen nucleus is. What Is A Result Of The Unequal Electron Sharing In A Water Molecule.
From dokumen.tips
(PPT) Properties of Water Covalent bonding sharing of electrons What Is A Result Of The Unequal Electron Sharing In A Water Molecule Unequal distribution of charge in a molecule. The unequal sharing of electrons gives the water molecule a slight negative charge near its oxygen atom and a slight positive charge near its. The unequal sharing of electrons between the atoms and the unsymmetrical shape of the molecule means that a water molecule has two. Water is a simple molecule consisting of. What Is A Result Of The Unequal Electron Sharing In A Water Molecule.
From slideplayer.com
Water ppt download What Is A Result Of The Unequal Electron Sharing In A Water Molecule A polar covalent bond is a covalent bond in which the atoms have an unequal attraction for electrons and so the sharing is. It makes it a polar molecule. Unequal distribution of charge in a molecule. In water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. The. What Is A Result Of The Unequal Electron Sharing In A Water Molecule.
From www.getbodysmart.com
Chemical Properties of Water GetBodySmart What Is A Result Of The Unequal Electron Sharing In A Water Molecule Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. It makes it a polar molecule. Because of the higher electronegativity of the oxygen atom, the bonds are polar. Unequal distribution of charge in a molecule. In water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons. What Is A Result Of The Unequal Electron Sharing In A Water Molecule.
From slidetodoc.com
QOD What is the difference between a covalent What Is A Result Of The Unequal Electron Sharing In A Water Molecule What does the unequal sharing of electrons within a water molecule make the water molecule? Unequal distribution of charge in a molecule. The unequal sharing of electrons between the atoms and the unsymmetrical shape of the molecule means that a water molecule has two. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms.. What Is A Result Of The Unequal Electron Sharing In A Water Molecule.
From slideplayer.com
Chapter 02 Lecture Outline ppt download What Is A Result Of The Unequal Electron Sharing In A Water Molecule Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Because of the higher electronegativity of the oxygen atom, the bonds are polar. It makes it a polar molecule. What is the result of these covalent. What does the unequal sharing of electrons within a water molecule make the water molecule? The unequal sharing. What Is A Result Of The Unequal Electron Sharing In A Water Molecule.
From www.chegg.com
Solved 1) In A Water Molecule. A) Unequal Sharing Of Elec... What Is A Result Of The Unequal Electron Sharing In A Water Molecule What does the unequal sharing of electrons within a water molecule make the water molecule? What is the result of these covalent. Unequal distribution of charge in a molecule. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Because of the higher electronegativity of the oxygen atom, the bonds are polar. It makes. What Is A Result Of The Unequal Electron Sharing In A Water Molecule.
From www.slideserve.com
PPT Chapter 3 Biochemistry PowerPoint Presentation, free download What Is A Result Of The Unequal Electron Sharing In A Water Molecule It makes it a polar molecule. The unequal sharing of electrons between the atoms and the unsymmetrical shape of the molecule means that a water molecule has two. Unequal distribution of charge in a molecule. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Because of the higher electronegativity of the oxygen atom,. What Is A Result Of The Unequal Electron Sharing In A Water Molecule.
From www.pinterest.com
Why Is Water a Polar Molecule? Water molecule, Molecular geometry What Is A Result Of The Unequal Electron Sharing In A Water Molecule What does the unequal sharing of electrons within a water molecule make the water molecule? The unequal sharing of electrons gives the water molecule a slight negative charge near its oxygen atom and a slight positive charge near its. Unequal distribution of charge in a molecule. Water is a simple molecule consisting of one oxygen atom bonded to two different. What Is A Result Of The Unequal Electron Sharing In A Water Molecule.
From slideplayer.com
Hydrologic Cycle, Properties of Water, Factors affecting Life in Water What Is A Result Of The Unequal Electron Sharing In A Water Molecule Because of the higher electronegativity of the oxygen atom, the bonds are polar. The unequal sharing of electrons gives the water molecule a slight negative charge near its oxygen atom and a slight positive charge near its. It makes it a polar molecule. Are the electrons in a water molecule shared equally? The unequal sharing of electrons between the atoms. What Is A Result Of The Unequal Electron Sharing In A Water Molecule.
From www.numerade.com
SOLVED 'Amoeba Sisters Video Selec 1. The molecule of water is What Is A Result Of The Unequal Electron Sharing In A Water Molecule Are the electrons in a water molecule shared equally? The unequal sharing of electrons between the atoms and the unsymmetrical shape of the molecule means that a water molecule has two. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Because of the higher electronegativity of the oxygen atom, the bonds are polar.. What Is A Result Of The Unequal Electron Sharing In A Water Molecule.
From slideplayer.com
Study Guide 3rd Quarter Exam. ppt download What Is A Result Of The Unequal Electron Sharing In A Water Molecule Unequal distribution of charge in a molecule. A polar covalent bond is a covalent bond in which the atoms have an unequal attraction for electrons and so the sharing is. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. What is the result of these covalent. The unequal sharing of electrons between the. What Is A Result Of The Unequal Electron Sharing In A Water Molecule.
From slideplayer.com
DR AMINA TARIQ BIOCHEMISTRY ppt download What Is A Result Of The Unequal Electron Sharing In A Water Molecule Are the electrons in a water molecule shared equally? A polar covalent bond is a covalent bond in which the atoms have an unequal attraction for electrons and so the sharing is. The unequal sharing of electrons between the atoms and the unsymmetrical shape of the molecule means that a water molecule has two. In water, each hydrogen nucleus is. What Is A Result Of The Unequal Electron Sharing In A Water Molecule.
From slideplayer.com
Faculty of medicine, U of D ppt download What Is A Result Of The Unequal Electron Sharing In A Water Molecule Because of the higher electronegativity of the oxygen atom, the bonds are polar. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. The unequal sharing of electrons gives the water molecule a slight negative charge near its oxygen atom and a slight positive charge near its. In water, each hydrogen nucleus is covalently. What Is A Result Of The Unequal Electron Sharing In A Water Molecule.
From www.slideserve.com
PPT Chemical Bonding PowerPoint Presentation, free download ID5674609 What Is A Result Of The Unequal Electron Sharing In A Water Molecule What is the result of these covalent. Unequal distribution of charge in a molecule. It makes it a polar molecule. The unequal sharing of electrons gives the water molecule a slight negative charge near its oxygen atom and a slight positive charge near its. Because of the higher electronegativity of the oxygen atom, the bonds are polar. Water is a. What Is A Result Of The Unequal Electron Sharing In A Water Molecule.
From slideplayer.com
Properties of Water Polar covalent bond unequal sharing of electrons What Is A Result Of The Unequal Electron Sharing In A Water Molecule Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. The unequal sharing of electrons gives the water molecule a slight negative charge near its oxygen atom and a slight positive charge near its. What does the unequal sharing of electrons within a water molecule make the water molecule? It makes it a polar. What Is A Result Of The Unequal Electron Sharing In A Water Molecule.
From biostudizz.weebly.com
WATER CHEMISTRY to Bio Stud... What Is A Result Of The Unequal Electron Sharing In A Water Molecule Because of the higher electronegativity of the oxygen atom, the bonds are polar. The unequal sharing of electrons gives the water molecule a slight negative charge near its oxygen atom and a slight positive charge near its. What does the unequal sharing of electrons within a water molecule make the water molecule? Water is a simple molecule consisting of one. What Is A Result Of The Unequal Electron Sharing In A Water Molecule.
From slideplayer.com
Properties of Water Polar covalent bond unequal sharing of electrons What Is A Result Of The Unequal Electron Sharing In A Water Molecule A polar covalent bond is a covalent bond in which the atoms have an unequal attraction for electrons and so the sharing is. What does the unequal sharing of electrons within a water molecule make the water molecule? The unequal sharing of electrons gives the water molecule a slight negative charge near its oxygen atom and a slight positive charge. What Is A Result Of The Unequal Electron Sharing In A Water Molecule.
From www.numerade.com
SOLVED The unequal sharing of electrons within a water molecule makes What Is A Result Of The Unequal Electron Sharing In A Water Molecule The unequal sharing of electrons gives the water molecule a slight negative charge near its oxygen atom and a slight positive charge near its. What is the result of these covalent. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. The unequal sharing of electrons between the atoms and the unsymmetrical shape of. What Is A Result Of The Unequal Electron Sharing In A Water Molecule.
From www.e-education.psu.edu
The Configuration of the Water Molecule EARTH 111 Water Science and What Is A Result Of The Unequal Electron Sharing In A Water Molecule Unequal distribution of charge in a molecule. What does the unequal sharing of electrons within a water molecule make the water molecule? Are the electrons in a water molecule shared equally? Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. It makes it a polar molecule. A polar covalent bond is a covalent. What Is A Result Of The Unequal Electron Sharing In A Water Molecule.
From slideplayer.com
Chapter 12 Chemical Bonding ppt download What Is A Result Of The Unequal Electron Sharing In A Water Molecule A polar covalent bond is a covalent bond in which the atoms have an unequal attraction for electrons and so the sharing is. It makes it a polar molecule. Unequal distribution of charge in a molecule. What does the unequal sharing of electrons within a water molecule make the water molecule? The unequal sharing of electrons between the atoms and. What Is A Result Of The Unequal Electron Sharing In A Water Molecule.
From slideplayer.com
Water Unit. ppt download What Is A Result Of The Unequal Electron Sharing In A Water Molecule The unequal sharing of electrons gives the water molecule a slight negative charge near its oxygen atom and a slight positive charge near its. Unequal distribution of charge in a molecule. The unequal sharing of electrons between the atoms and the unsymmetrical shape of the molecule means that a water molecule has two. A polar covalent bond is a covalent. What Is A Result Of The Unequal Electron Sharing In A Water Molecule.
From philschatz.com
Chemical Bonds · Anatomy and Physiology What Is A Result Of The Unequal Electron Sharing In A Water Molecule The unequal sharing of electrons between the atoms and the unsymmetrical shape of the molecule means that a water molecule has two. What does the unequal sharing of electrons within a water molecule make the water molecule? Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Are the electrons in a water molecule. What Is A Result Of The Unequal Electron Sharing In A Water Molecule.
From www.slideserve.com
PPT Section 1 Atoms, Bonding, and the Periodic Table PowerPoint What Is A Result Of The Unequal Electron Sharing In A Water Molecule In water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. The unequal sharing of electrons gives the water molecule a slight negative charge near its oxygen atom and a slight positive charge near its. Because of the higher electronegativity of the oxygen atom, the bonds are polar.. What Is A Result Of The Unequal Electron Sharing In A Water Molecule.
From www.studocu.com
BILD 1 homework 1 A water molecule has an unequal sharing of What Is A Result Of The Unequal Electron Sharing In A Water Molecule What does the unequal sharing of electrons within a water molecule make the water molecule? Unequal distribution of charge in a molecule. It makes it a polar molecule. The unequal sharing of electrons gives the water molecule a slight negative charge near its oxygen atom and a slight positive charge near its. What is the result of these covalent. The. What Is A Result Of The Unequal Electron Sharing In A Water Molecule.
From www.slideserve.com
PPT Bioquímica I Química Biológica I PowerPoint Presentation, free What Is A Result Of The Unequal Electron Sharing In A Water Molecule Because of the higher electronegativity of the oxygen atom, the bonds are polar. It makes it a polar molecule. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. A polar covalent bond is a covalent bond in which the atoms have an unequal attraction for electrons and so the sharing is. Unequal distribution. What Is A Result Of The Unequal Electron Sharing In A Water Molecule.
From taylorsciencegeeks.weebly.com
The Water Molecule Science News What Is A Result Of The Unequal Electron Sharing In A Water Molecule Unequal distribution of charge in a molecule. What is the result of these covalent. Are the electrons in a water molecule shared equally? What does the unequal sharing of electrons within a water molecule make the water molecule? The unequal sharing of electrons gives the water molecule a slight negative charge near its oxygen atom and a slight positive charge. What Is A Result Of The Unequal Electron Sharing In A Water Molecule.
From www.medicalsciencenavigator.com
Physiology of Cell Signaling What Is A Result Of The Unequal Electron Sharing In A Water Molecule Are the electrons in a water molecule shared equally? What does the unequal sharing of electrons within a water molecule make the water molecule? What is the result of these covalent. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. A polar covalent bond is a covalent bond in which the atoms have. What Is A Result Of The Unequal Electron Sharing In A Water Molecule.
From www.numerade.com
SOLVED 'Amoeba Sisters Video Selec 1. The molecule of water is What Is A Result Of The Unequal Electron Sharing In A Water Molecule Are the electrons in a water molecule shared equally? Because of the higher electronegativity of the oxygen atom, the bonds are polar. What is the result of these covalent. The unequal sharing of electrons gives the water molecule a slight negative charge near its oxygen atom and a slight positive charge near its. Water is a simple molecule consisting of. What Is A Result Of The Unequal Electron Sharing In A Water Molecule.