Chemical Formula For Baking Soda And Vinegar . Each molecule of baking soda contains a sodium atom, a hydrogen atom, an oxygen atom, and a carbon dioxide molecule. Learn how baking soda and vinegar react to form carbon dioxide, water and ions. The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic. This formula represents the composition of vinegar, which consists of. Vinegar contains acetic acid, each molecule of which contains a hydrogen atom, and an acetate ion. Explore fun science experiments with fizzy. This chemistry video tutorial discusses the reaction between baking soda and vinegar. Baking soda is sodium bicarbonate: The combination of baking soda (sodium bicarbonate, nahco3) and vinegar (acetic acid, ch3cooh) results in a chemical reaction that. The chemical formula for vinegar is **ch3cooh**.
from www.tessshebaylo.com
This chemistry video tutorial discusses the reaction between baking soda and vinegar. Each molecule of baking soda contains a sodium atom, a hydrogen atom, an oxygen atom, and a carbon dioxide molecule. This formula represents the composition of vinegar, which consists of. Baking soda is sodium bicarbonate: The chemical formula for vinegar is **ch3cooh**. Learn how baking soda and vinegar react to form carbon dioxide, water and ions. The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic. The combination of baking soda (sodium bicarbonate, nahco3) and vinegar (acetic acid, ch3cooh) results in a chemical reaction that. Explore fun science experiments with fizzy. Vinegar contains acetic acid, each molecule of which contains a hydrogen atom, and an acetate ion.
Chemical Equation For Baking Soda Citric Acid And Water Tessshebaylo
Chemical Formula For Baking Soda And Vinegar Each molecule of baking soda contains a sodium atom, a hydrogen atom, an oxygen atom, and a carbon dioxide molecule. Vinegar contains acetic acid, each molecule of which contains a hydrogen atom, and an acetate ion. The combination of baking soda (sodium bicarbonate, nahco3) and vinegar (acetic acid, ch3cooh) results in a chemical reaction that. Learn how baking soda and vinegar react to form carbon dioxide, water and ions. Explore fun science experiments with fizzy. Each molecule of baking soda contains a sodium atom, a hydrogen atom, an oxygen atom, and a carbon dioxide molecule. This chemistry video tutorial discusses the reaction between baking soda and vinegar. The chemical formula for vinegar is **ch3cooh**. Baking soda is sodium bicarbonate: This formula represents the composition of vinegar, which consists of. The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic.
From readingandwritingprojectcom.web.fc2.com
baking soda and vinegar reaction equation Chemical Formula For Baking Soda And Vinegar Learn how baking soda and vinegar react to form carbon dioxide, water and ions. The chemical formula for vinegar is **ch3cooh**. Baking soda is sodium bicarbonate: This chemistry video tutorial discusses the reaction between baking soda and vinegar. This formula represents the composition of vinegar, which consists of. Vinegar contains acetic acid, each molecule of which contains a hydrogen atom,. Chemical Formula For Baking Soda And Vinegar.
From www.vectorstock.com
Science experiment with baking soda and vinegar Vector Image Chemical Formula For Baking Soda And Vinegar The combination of baking soda (sodium bicarbonate, nahco3) and vinegar (acetic acid, ch3cooh) results in a chemical reaction that. Each molecule of baking soda contains a sodium atom, a hydrogen atom, an oxygen atom, and a carbon dioxide molecule. The chemical formula for vinegar is **ch3cooh**. The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid). Chemical Formula For Baking Soda And Vinegar.
From colouremployer8.gitlab.io
Unique Chemical Equation Of Vinegar And Baking Soda Electrostatic Notes Class 12 Pdf Chemical Formula For Baking Soda And Vinegar This chemistry video tutorial discusses the reaction between baking soda and vinegar. Learn how baking soda and vinegar react to form carbon dioxide, water and ions. The chemical formula for vinegar is **ch3cooh**. The combination of baking soda (sodium bicarbonate, nahco3) and vinegar (acetic acid, ch3cooh) results in a chemical reaction that. Each molecule of baking soda contains a sodium. Chemical Formula For Baking Soda And Vinegar.
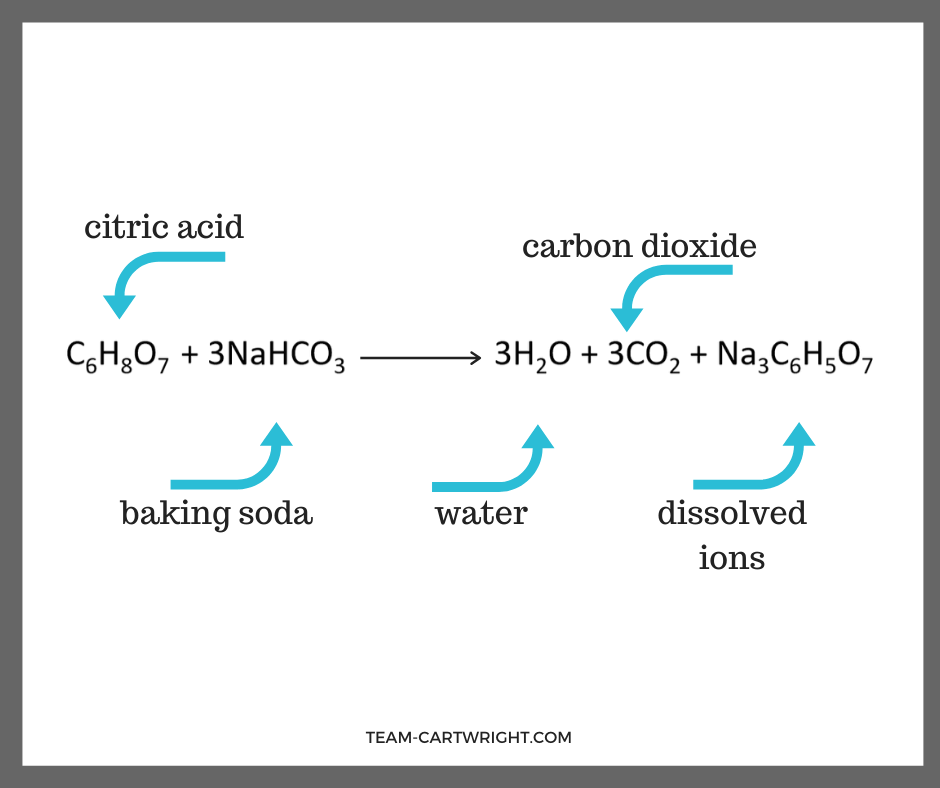
From team-cartwright.com
Fizzy Heart Science Experiment Team Cartwright Chemical Formula For Baking Soda And Vinegar This formula represents the composition of vinegar, which consists of. Explore fun science experiments with fizzy. The combination of baking soda (sodium bicarbonate, nahco3) and vinegar (acetic acid, ch3cooh) results in a chemical reaction that. The chemical formula for vinegar is **ch3cooh**. Baking soda is sodium bicarbonate: This chemistry video tutorial discusses the reaction between baking soda and vinegar. The. Chemical Formula For Baking Soda And Vinegar.
From www.slideserve.com
PPT Baking Soda and Vinegar Limiting Reactant Lab PowerPoint Presentation ID2882558 Chemical Formula For Baking Soda And Vinegar This chemistry video tutorial discusses the reaction between baking soda and vinegar. The combination of baking soda (sodium bicarbonate, nahco3) and vinegar (acetic acid, ch3cooh) results in a chemical reaction that. Each molecule of baking soda contains a sodium atom, a hydrogen atom, an oxygen atom, and a carbon dioxide molecule. The overall chemical reaction between baking soda (sodium bicarbonate). Chemical Formula For Baking Soda And Vinegar.
From www.youtube.com
Baking Soda and Vinegar Equation and Reaction YouTube Chemical Formula For Baking Soda And Vinegar Baking soda is sodium bicarbonate: Each molecule of baking soda contains a sodium atom, a hydrogen atom, an oxygen atom, and a carbon dioxide molecule. This chemistry video tutorial discusses the reaction between baking soda and vinegar. This formula represents the composition of vinegar, which consists of. The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic. Chemical Formula For Baking Soda And Vinegar.
From www.slideserve.com
PPT Baking Soda and Vinegar Limiting Reactant Lab PowerPoint Presentation ID6793262 Chemical Formula For Baking Soda And Vinegar The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic. Learn how baking soda and vinegar react to form carbon dioxide, water and ions. This formula represents the composition of vinegar, which consists of. Each molecule of baking soda contains a sodium. Chemical Formula For Baking Soda And Vinegar.
From chemistry291.blogspot.com
Baking Soda and Vinegar Chemical Reaction ExplanationNaHCO3 + CH3COOH Chemical Formula For Baking Soda And Vinegar This chemistry video tutorial discusses the reaction between baking soda and vinegar. The combination of baking soda (sodium bicarbonate, nahco3) and vinegar (acetic acid, ch3cooh) results in a chemical reaction that. Explore fun science experiments with fizzy. Baking soda is sodium bicarbonate: Each molecule of baking soda contains a sodium atom, a hydrogen atom, an oxygen atom, and a carbon. Chemical Formula For Baking Soda And Vinegar.
From www.pinterest.com
Know the Equation for the Baking Soda and Vinegar Reaction Baking soda, Science activities, Soda Chemical Formula For Baking Soda And Vinegar Learn how baking soda and vinegar react to form carbon dioxide, water and ions. Baking soda is sodium bicarbonate: Vinegar contains acetic acid, each molecule of which contains a hydrogen atom, and an acetate ion. This formula represents the composition of vinegar, which consists of. The chemical formula for vinegar is **ch3cooh**. Each molecule of baking soda contains a sodium. Chemical Formula For Baking Soda And Vinegar.
From bakingsodaandvinegarbonding.weebly.com
Bonding Process Chemical Formula For Baking Soda And Vinegar The combination of baking soda (sodium bicarbonate, nahco3) and vinegar (acetic acid, ch3cooh) results in a chemical reaction that. The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic. Explore fun science experiments with fizzy. Learn how baking soda and vinegar react. Chemical Formula For Baking Soda And Vinegar.
From www.numerade.com
SOLVED Baking soda and vinegar react according to the following chemical equation NaHCO3 Chemical Formula For Baking Soda And Vinegar The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic. Baking soda is sodium bicarbonate: Vinegar contains acetic acid, each molecule of which contains a hydrogen atom, and an acetate ion. This formula represents the composition of vinegar, which consists of. The. Chemical Formula For Baking Soda And Vinegar.
From www.numerade.com
SOLVED The chemical equation for the reaction of baking soda (sodium bicarbonate, nahco3) and Chemical Formula For Baking Soda And Vinegar Each molecule of baking soda contains a sodium atom, a hydrogen atom, an oxygen atom, and a carbon dioxide molecule. Learn how baking soda and vinegar react to form carbon dioxide, water and ions. Baking soda is sodium bicarbonate: This chemistry video tutorial discusses the reaction between baking soda and vinegar. The overall chemical reaction between baking soda (sodium bicarbonate). Chemical Formula For Baking Soda And Vinegar.
From www.numerade.com
SOLVED The chemical equation for the reaction of baking soda (sodium bicarbonate, NaHCO3) and Chemical Formula For Baking Soda And Vinegar Vinegar contains acetic acid, each molecule of which contains a hydrogen atom, and an acetate ion. The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic. Learn how baking soda and vinegar react to form carbon dioxide, water and ions. The combination. Chemical Formula For Baking Soda And Vinegar.
From katierossparks.blogspot.com
Baking Soda Chemical Formula KatierosSparks Chemical Formula For Baking Soda And Vinegar Each molecule of baking soda contains a sodium atom, a hydrogen atom, an oxygen atom, and a carbon dioxide molecule. Baking soda is sodium bicarbonate: The chemical formula for vinegar is **ch3cooh**. Vinegar contains acetic acid, each molecule of which contains a hydrogen atom, and an acetate ion. This chemistry video tutorial discusses the reaction between baking soda and vinegar.. Chemical Formula For Baking Soda And Vinegar.
From www.congress-intercultural.eu
Sodium Bicarbonate Molecule, Known As Baking Structural, 42 OFF Chemical Formula For Baking Soda And Vinegar The combination of baking soda (sodium bicarbonate, nahco3) and vinegar (acetic acid, ch3cooh) results in a chemical reaction that. Explore fun science experiments with fizzy. Baking soda is sodium bicarbonate: The chemical formula for vinegar is **ch3cooh**. Vinegar contains acetic acid, each molecule of which contains a hydrogen atom, and an acetate ion. This formula represents the composition of vinegar,. Chemical Formula For Baking Soda And Vinegar.
From tikalon.com
Tikalon Blog by Dev Gualtieri Chemical Formula For Baking Soda And Vinegar Vinegar contains acetic acid, each molecule of which contains a hydrogen atom, and an acetate ion. This formula represents the composition of vinegar, which consists of. This chemistry video tutorial discusses the reaction between baking soda and vinegar. The chemical formula for vinegar is **ch3cooh**. The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is. Chemical Formula For Baking Soda And Vinegar.
From signalticket9.pythonanywhere.com
Marvelous Vinegar Plus Baking Soda Chemical Equation Balanced For And Chemical Formula For Baking Soda And Vinegar Baking soda is sodium bicarbonate: The combination of baking soda (sodium bicarbonate, nahco3) and vinegar (acetic acid, ch3cooh) results in a chemical reaction that. Learn how baking soda and vinegar react to form carbon dioxide, water and ions. The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts. Chemical Formula For Baking Soda And Vinegar.
From www.slideserve.com
PPT Baking Soda/Vinegar Stoichiometry Lab PowerPoint Presentation ID203234 Chemical Formula For Baking Soda And Vinegar The chemical formula for vinegar is **ch3cooh**. Each molecule of baking soda contains a sodium atom, a hydrogen atom, an oxygen atom, and a carbon dioxide molecule. The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic. Learn how baking soda and. Chemical Formula For Baking Soda And Vinegar.
From asideload7.gitlab.io
Amazing Balanced Chemical Equation Of Vinegar And Baking Soda Differentiation Formulas List For Chemical Formula For Baking Soda And Vinegar Each molecule of baking soda contains a sodium atom, a hydrogen atom, an oxygen atom, and a carbon dioxide molecule. Explore fun science experiments with fizzy. Baking soda is sodium bicarbonate: Learn how baking soda and vinegar react to form carbon dioxide, water and ions. This chemistry video tutorial discusses the reaction between baking soda and vinegar. The overall chemical. Chemical Formula For Baking Soda And Vinegar.
From slideplayer.com
Daily Science What’s the rule for how many electrons can fit in each orbital 13? What does Chemical Formula For Baking Soda And Vinegar Learn how baking soda and vinegar react to form carbon dioxide, water and ions. This formula represents the composition of vinegar, which consists of. The chemical formula for vinegar is **ch3cooh**. Vinegar contains acetic acid, each molecule of which contains a hydrogen atom, and an acetate ion. The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic. Chemical Formula For Baking Soda And Vinegar.
From shotprofessional22.gitlab.io
Supreme Baking Soda And Vinegar Word Equation C Formula Physics Chemical Formula For Baking Soda And Vinegar Baking soda is sodium bicarbonate: Vinegar contains acetic acid, each molecule of which contains a hydrogen atom, and an acetate ion. Explore fun science experiments with fizzy. The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic. This chemistry video tutorial discusses. Chemical Formula For Baking Soda And Vinegar.
From ar.inspiredpencil.com
Vinegar And Baking Soda Chemical Reaction Chemical Formula For Baking Soda And Vinegar The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic. Explore fun science experiments with fizzy. This chemistry video tutorial discusses the reaction between baking soda and vinegar. The chemical formula for vinegar is **ch3cooh**. Learn how baking soda and vinegar react. Chemical Formula For Baking Soda And Vinegar.
From classschooldairywoman.z21.web.core.windows.net
Baking Soda And Vinegar Experiment Chemical Formula For Baking Soda And Vinegar Vinegar contains acetic acid, each molecule of which contains a hydrogen atom, and an acetate ion. Explore fun science experiments with fizzy. Each molecule of baking soda contains a sodium atom, a hydrogen atom, an oxygen atom, and a carbon dioxide molecule. This chemistry video tutorial discusses the reaction between baking soda and vinegar. Baking soda is sodium bicarbonate: This. Chemical Formula For Baking Soda And Vinegar.
From exotjzliw.blob.core.windows.net
Vinegar + Baking Soda Chemical Equation at Allen Oneal blog Chemical Formula For Baking Soda And Vinegar Baking soda is sodium bicarbonate: Vinegar contains acetic acid, each molecule of which contains a hydrogen atom, and an acetate ion. This formula represents the composition of vinegar, which consists of. Learn how baking soda and vinegar react to form carbon dioxide, water and ions. The combination of baking soda (sodium bicarbonate, nahco3) and vinegar (acetic acid, ch3cooh) results in. Chemical Formula For Baking Soda And Vinegar.
From www.coursehero.com
[Solved] 6. The equation for the reaction between baking soda and vinegar... Course Hero Chemical Formula For Baking Soda And Vinegar This chemistry video tutorial discusses the reaction between baking soda and vinegar. The chemical formula for vinegar is **ch3cooh**. Learn how baking soda and vinegar react to form carbon dioxide, water and ions. Each molecule of baking soda contains a sodium atom, a hydrogen atom, an oxygen atom, and a carbon dioxide molecule. This formula represents the composition of vinegar,. Chemical Formula For Baking Soda And Vinegar.
From www.saubhaya.com
What Is The Chemical Makeup Of Vinegar Saubhaya Makeup Chemical Formula For Baking Soda And Vinegar This chemistry video tutorial discusses the reaction between baking soda and vinegar. Vinegar contains acetic acid, each molecule of which contains a hydrogen atom, and an acetate ion. Each molecule of baking soda contains a sodium atom, a hydrogen atom, an oxygen atom, and a carbon dioxide molecule. The chemical formula for vinegar is **ch3cooh**. Learn how baking soda and. Chemical Formula For Baking Soda And Vinegar.
From www.youtube.com
Baking Soda and Vinegar Chemical Reaction (EXPERIMENT EXPLAINED) YouTube Chemical Formula For Baking Soda And Vinegar The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic. Vinegar contains acetic acid, each molecule of which contains a hydrogen atom, and an acetate ion. Baking soda is sodium bicarbonate: Each molecule of baking soda contains a sodium atom, a hydrogen. Chemical Formula For Baking Soda And Vinegar.
From exotjzliw.blob.core.windows.net
Vinegar + Baking Soda Chemical Equation at Allen Oneal blog Chemical Formula For Baking Soda And Vinegar This formula represents the composition of vinegar, which consists of. Explore fun science experiments with fizzy. The combination of baking soda (sodium bicarbonate, nahco3) and vinegar (acetic acid, ch3cooh) results in a chemical reaction that. The chemical formula for vinegar is **ch3cooh**. Vinegar contains acetic acid, each molecule of which contains a hydrogen atom, and an acetate ion. Learn how. Chemical Formula For Baking Soda And Vinegar.
From treatybottle13.pythonanywhere.com
Amazing Word Equation For Baking Soda And Vinegar What Is Magnitude Of Velocity Chemical Formula For Baking Soda And Vinegar The chemical formula for vinegar is **ch3cooh**. Vinegar contains acetic acid, each molecule of which contains a hydrogen atom, and an acetate ion. Explore fun science experiments with fizzy. Each molecule of baking soda contains a sodium atom, a hydrogen atom, an oxygen atom, and a carbon dioxide molecule. The combination of baking soda (sodium bicarbonate, nahco3) and vinegar (acetic. Chemical Formula For Baking Soda And Vinegar.
From www.saubhaya.com
What Chemicals Make Up Baking Soda Saubhaya Makeup Chemical Formula For Baking Soda And Vinegar Each molecule of baking soda contains a sodium atom, a hydrogen atom, an oxygen atom, and a carbon dioxide molecule. The chemical formula for vinegar is **ch3cooh**. Explore fun science experiments with fizzy. This chemistry video tutorial discusses the reaction between baking soda and vinegar. The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is. Chemical Formula For Baking Soda And Vinegar.
From shotprofessional22.gitlab.io
Ideal What Is The Balanced Chemical Equation For Vinegar And Baking Soda Collision Theory Answer Key Chemical Formula For Baking Soda And Vinegar Vinegar contains acetic acid, each molecule of which contains a hydrogen atom, and an acetate ion. Baking soda is sodium bicarbonate: The combination of baking soda (sodium bicarbonate, nahco3) and vinegar (acetic acid, ch3cooh) results in a chemical reaction that. This chemistry video tutorial discusses the reaction between baking soda and vinegar. The overall chemical reaction between baking soda (sodium. Chemical Formula For Baking Soda And Vinegar.
From www.thekitchn.com
Why You Shouldn't Mix Baking Soda and Vinegar for Cleaning The Kitchn Chemical Formula For Baking Soda And Vinegar This formula represents the composition of vinegar, which consists of. Learn how baking soda and vinegar react to form carbon dioxide, water and ions. The chemical formula for vinegar is **ch3cooh**. The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic. This. Chemical Formula For Baking Soda And Vinegar.
From www.ingridscience.ca
Baking soda and vinegar ingridscience.ca Chemical Formula For Baking Soda And Vinegar This formula represents the composition of vinegar, which consists of. The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic. Explore fun science experiments with fizzy. This chemistry video tutorial discusses the reaction between baking soda and vinegar. Vinegar contains acetic acid,. Chemical Formula For Baking Soda And Vinegar.
From chemicalforfo.blogspot.com
Chemical Formula Of Baking Soda And Vinegar Reaction Chemical Formula Info Chemical Formula For Baking Soda And Vinegar This formula represents the composition of vinegar, which consists of. The chemical formula for vinegar is **ch3cooh**. This chemistry video tutorial discusses the reaction between baking soda and vinegar. The combination of baking soda (sodium bicarbonate, nahco3) and vinegar (acetic acid, ch3cooh) results in a chemical reaction that. Learn how baking soda and vinegar react to form carbon dioxide, water. Chemical Formula For Baking Soda And Vinegar.
From www.tessshebaylo.com
Chemical Equation For Baking Soda Citric Acid And Water Tessshebaylo Chemical Formula For Baking Soda And Vinegar Baking soda is sodium bicarbonate: This formula represents the composition of vinegar, which consists of. Explore fun science experiments with fizzy. The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic. This chemistry video tutorial discusses the reaction between baking soda and. Chemical Formula For Baking Soda And Vinegar.