Spectrophotometry Absorbance Difference . The instrument operates by passing a beam of. spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as. discover how optical density (od), absorbance, and transmittance interplay to determine concentration in. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a sample, and can be. spectrophotometers are instruments designed to detect the amount of light energy that is absorbed or transmitted by molecules dissolved in a solution. Absorbance is a measure of how much infrared radiation is absorbed by a sample at a particular. what is the %t for a sample if its absorbance is 1.27? in spectrophotometry, we either measure the absorbance or emission of emr after its interaction with matter. a spectrophotometer measures the amount of light that a sample absorbs.
from www.analyzetest.com
in spectrophotometry, we either measure the absorbance or emission of emr after its interaction with matter. The instrument operates by passing a beam of. what is the %t for a sample if its absorbance is 1.27? spectrophotometers are instruments designed to detect the amount of light energy that is absorbed or transmitted by molecules dissolved in a solution. Absorbance is a measure of how much infrared radiation is absorbed by a sample at a particular. discover how optical density (od), absorbance, and transmittance interplay to determine concentration in. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a sample, and can be. a spectrophotometer measures the amount of light that a sample absorbs. spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as.
A to Z of UVVis spectroscopy interpretation
Spectrophotometry Absorbance Difference in spectrophotometry, we either measure the absorbance or emission of emr after its interaction with matter. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a sample, and can be. spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as. a spectrophotometer measures the amount of light that a sample absorbs. what is the %t for a sample if its absorbance is 1.27? spectrophotometers are instruments designed to detect the amount of light energy that is absorbed or transmitted by molecules dissolved in a solution. in spectrophotometry, we either measure the absorbance or emission of emr after its interaction with matter. The instrument operates by passing a beam of. discover how optical density (od), absorbance, and transmittance interplay to determine concentration in. Absorbance is a measure of how much infrared radiation is absorbed by a sample at a particular.
From dokumen.tips
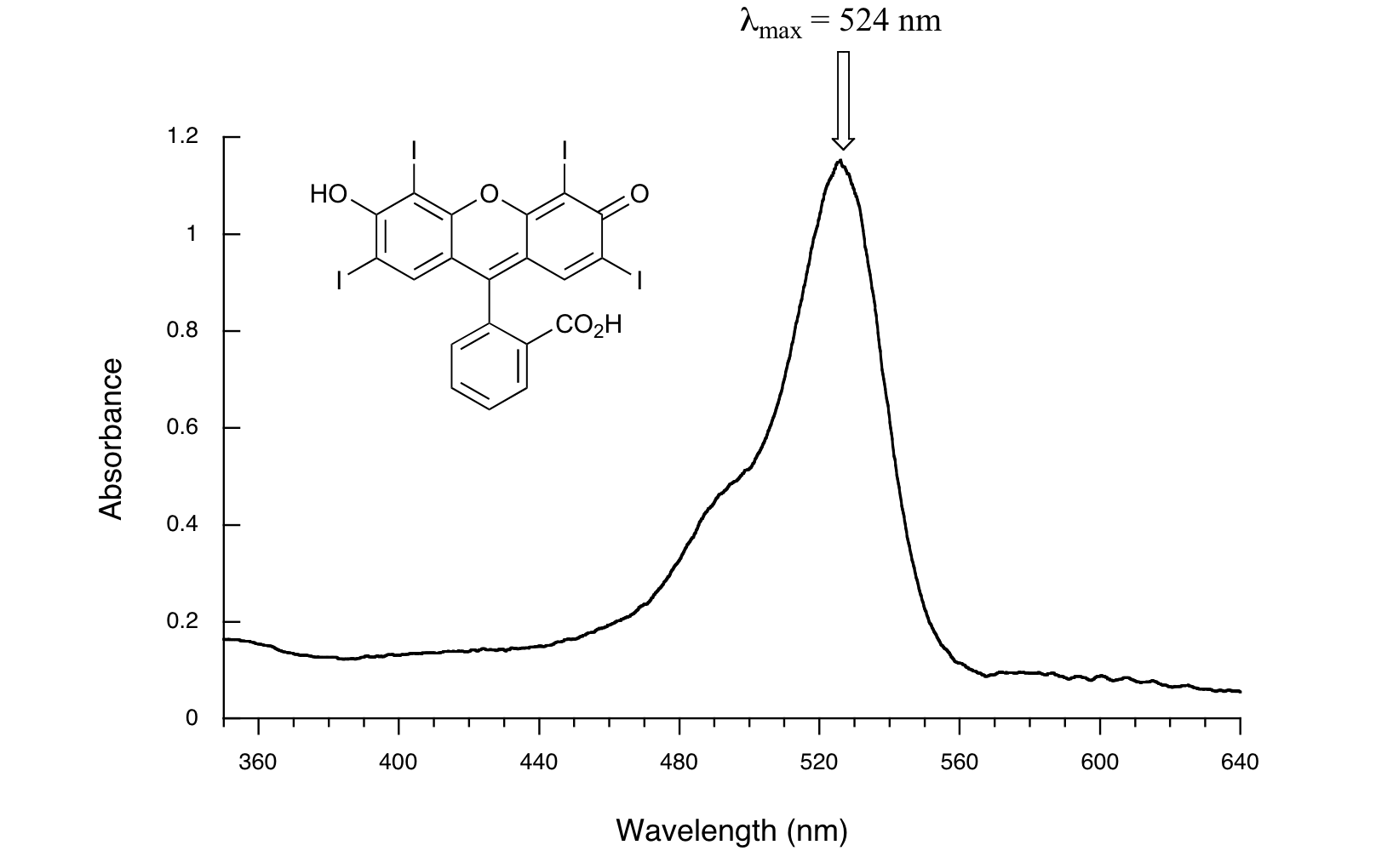
(PDF) Differencederivative absorbance spectrophotometry as a technique Spectrophotometry Absorbance Difference A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a sample, and can be. Absorbance is a measure of how much infrared radiation is absorbed by a sample at a particular. spectrophotometers are instruments designed to detect the amount of light energy that is absorbed or transmitted by molecules dissolved. Spectrophotometry Absorbance Difference.
From exonciqri.blob.core.windows.net
Examples For Uv Spectroscopy at Molly Garrison blog Spectrophotometry Absorbance Difference spectrophotometers are instruments designed to detect the amount of light energy that is absorbed or transmitted by molecules dissolved in a solution. The instrument operates by passing a beam of. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a sample, and can be. spectrophotometry is a method to. Spectrophotometry Absorbance Difference.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download Spectrophotometry Absorbance Difference A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a sample, and can be. discover how optical density (od), absorbance, and transmittance interplay to determine concentration in. The instrument operates by passing a beam of. spectrophotometry is a method to measure how much a chemical substance absorbs light by. Spectrophotometry Absorbance Difference.
From derangedphysiology.com
Absorption spectroscopy of haemoglobin species Deranged Physiology Spectrophotometry Absorbance Difference The instrument operates by passing a beam of. spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as. discover how optical density (od), absorbance, and transmittance interplay to determine concentration in. spectrophotometers are instruments designed to detect the amount of light energy that is absorbed or transmitted. Spectrophotometry Absorbance Difference.
From dxopruans.blob.core.windows.net
How To Calculate Absorbance In Uv Spectrophotometer at Jason Ward blog Spectrophotometry Absorbance Difference in spectrophotometry, we either measure the absorbance or emission of emr after its interaction with matter. spectrophotometers are instruments designed to detect the amount of light energy that is absorbed or transmitted by molecules dissolved in a solution. what is the %t for a sample if its absorbance is 1.27? a spectrophotometer measures the amount of. Spectrophotometry Absorbance Difference.
From dxoxtajwq.blob.core.windows.net
Transmission Vs Absorption at Cody ber blog Spectrophotometry Absorbance Difference spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a sample, and can be. Absorbance is a measure of how much infrared radiation is absorbed by a sample at a. Spectrophotometry Absorbance Difference.
From chem.libretexts.org
13.21.1.1 Some Uses of UV/Vis Spectroscopy Chemistry LibreTexts Spectrophotometry Absorbance Difference discover how optical density (od), absorbance, and transmittance interplay to determine concentration in. spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as. in spectrophotometry, we either measure the absorbance or emission of emr after its interaction with matter. The instrument operates by passing a beam of.. Spectrophotometry Absorbance Difference.
From www.analyzetest.com
A to Z of UVVis spectroscopy interpretation Spectrophotometry Absorbance Difference in spectrophotometry, we either measure the absorbance or emission of emr after its interaction with matter. The instrument operates by passing a beam of. spectrophotometers are instruments designed to detect the amount of light energy that is absorbed or transmitted by molecules dissolved in a solution. a spectrophotometer measures the amount of light that a sample absorbs.. Spectrophotometry Absorbance Difference.
From www.differencebetween.com
Difference Between Colorimetry and Spectrophotometry Compare the Spectrophotometry Absorbance Difference spectrophotometers are instruments designed to detect the amount of light energy that is absorbed or transmitted by molecules dissolved in a solution. in spectrophotometry, we either measure the absorbance or emission of emr after its interaction with matter. a spectrophotometer measures the amount of light that a sample absorbs. discover how optical density (od), absorbance, and. Spectrophotometry Absorbance Difference.
From www.researchgate.net
Normalized absorbance and emission intensity spectra versus wavelength Spectrophotometry Absorbance Difference Absorbance is a measure of how much infrared radiation is absorbed by a sample at a particular. what is the %t for a sample if its absorbance is 1.27? in spectrophotometry, we either measure the absorbance or emission of emr after its interaction with matter. a spectrophotometer measures the amount of light that a sample absorbs. A. Spectrophotometry Absorbance Difference.
From quizdboenometers.z13.web.core.windows.net
What Is Absorbance Units Spectrophotometry Absorbance Difference a spectrophotometer measures the amount of light that a sample absorbs. what is the %t for a sample if its absorbance is 1.27? spectrophotometers are instruments designed to detect the amount of light energy that is absorbed or transmitted by molecules dissolved in a solution. in spectrophotometry, we either measure the absorbance or emission of emr. Spectrophotometry Absorbance Difference.
From justledus.com
Photoreceptors and Spectral Absorption Just Led Us Spectrophotometry Absorbance Difference in spectrophotometry, we either measure the absorbance or emission of emr after its interaction with matter. discover how optical density (od), absorbance, and transmittance interplay to determine concentration in. what is the %t for a sample if its absorbance is 1.27? spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring. Spectrophotometry Absorbance Difference.
From chem.libretexts.org
4.3 Ultraviolet and visible spectroscopy Chemistry LibreTexts Spectrophotometry Absorbance Difference spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as. The instrument operates by passing a beam of. spectrophotometers are instruments designed to detect the amount of light energy that is absorbed or transmitted by molecules dissolved in a solution. a spectrophotometer measures the amount of light. Spectrophotometry Absorbance Difference.
From www.youtube.com
Spectroscopy part 3 Transmittance and Absorbance YouTube Spectrophotometry Absorbance Difference Absorbance is a measure of how much infrared radiation is absorbed by a sample at a particular. spectrophotometers are instruments designed to detect the amount of light energy that is absorbed or transmitted by molecules dissolved in a solution. spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light. Spectrophotometry Absorbance Difference.
From chem.libretexts.org
10.6 Photoluminescence Spectroscopy Chemistry LibreTexts Spectrophotometry Absorbance Difference A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a sample, and can be. in spectrophotometry, we either measure the absorbance or emission of emr after its interaction with matter. The instrument operates by passing a beam of. a spectrophotometer measures the amount of light that a sample absorbs.. Spectrophotometry Absorbance Difference.
From exohaxrbd.blob.core.windows.net
Differences Between Mass Spectrometry And Infrared Spectroscopy at Spectrophotometry Absorbance Difference spectrophotometers are instruments designed to detect the amount of light energy that is absorbed or transmitted by molecules dissolved in a solution. The instrument operates by passing a beam of. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a sample, and can be. Absorbance is a measure of how. Spectrophotometry Absorbance Difference.
From schematicgonzina4e.z13.web.core.windows.net
Atomic Absorption Spectroscopy Schematic Diagram Spectrophotometry Absorbance Difference Absorbance is a measure of how much infrared radiation is absorbed by a sample at a particular. a spectrophotometer measures the amount of light that a sample absorbs. The instrument operates by passing a beam of. spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as. discover. Spectrophotometry Absorbance Difference.
From www.scribd.com
Introduction To Spectrophotometry PDF Spectrophotometry Absorbance Spectrophotometry Absorbance Difference spectrophotometers are instruments designed to detect the amount of light energy that is absorbed or transmitted by molecules dissolved in a solution. spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as. in spectrophotometry, we either measure the absorbance or emission of emr after its interaction with. Spectrophotometry Absorbance Difference.
From chemwiki.ucdavis.edu
Spectrophotometry Chemwiki Spectrophotometry Absorbance Difference discover how optical density (od), absorbance, and transmittance interplay to determine concentration in. what is the %t for a sample if its absorbance is 1.27? A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a sample, and can be. Absorbance is a measure of how much infrared radiation is. Spectrophotometry Absorbance Difference.
From webbtelescope.org
Spectroscopy 101 Types of Spectra and Spectroscopy b Spectrophotometry Absorbance Difference spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a sample, and can be. The instrument operates by passing a beam of. Absorbance is a measure of how much infrared. Spectrophotometry Absorbance Difference.
From exoggmvfx.blob.core.windows.net
Examples Of Absorption Spectroscopy at Drew Humbert blog Spectrophotometry Absorbance Difference Absorbance is a measure of how much infrared radiation is absorbed by a sample at a particular. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a sample, and can be. The instrument operates by passing a beam of. a spectrophotometer measures the amount of light that a sample absorbs.. Spectrophotometry Absorbance Difference.
From derangedphysiology.com
Absorption spectroscopy of haemoglobin species Deranged Physiology Spectrophotometry Absorbance Difference in spectrophotometry, we either measure the absorbance or emission of emr after its interaction with matter. Absorbance is a measure of how much infrared radiation is absorbed by a sample at a particular. spectrophotometers are instruments designed to detect the amount of light energy that is absorbed or transmitted by molecules dissolved in a solution. A spectrophotometer in. Spectrophotometry Absorbance Difference.
From www.chegg.com
Solved Spectrophotometers are used to measure the absorbance Spectrophotometry Absorbance Difference Absorbance is a measure of how much infrared radiation is absorbed by a sample at a particular. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a sample, and can be. spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light. Spectrophotometry Absorbance Difference.
From www.linquip.com
What Wikipedia Can’t Tell You About How Does a Spectrophotometer Work Spectrophotometry Absorbance Difference spectrophotometers are instruments designed to detect the amount of light energy that is absorbed or transmitted by molecules dissolved in a solution. in spectrophotometry, we either measure the absorbance or emission of emr after its interaction with matter. a spectrophotometer measures the amount of light that a sample absorbs. spectrophotometry is a method to measure how. Spectrophotometry Absorbance Difference.
From namrataheda.blogspot.com
B for Biology Spectrophotometry Atomic Absorption Spectrophotometry Spectrophotometry Absorbance Difference A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a sample, and can be. spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as. The instrument operates by passing a beam of. a spectrophotometer measures the amount of light. Spectrophotometry Absorbance Difference.
From www.odinity.com
Bromothymol Blue Spectrophotometry Report & Experiment Spectrophotometry Absorbance Difference a spectrophotometer measures the amount of light that a sample absorbs. in spectrophotometry, we either measure the absorbance or emission of emr after its interaction with matter. The instrument operates by passing a beam of. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a sample, and can be.. Spectrophotometry Absorbance Difference.
From www.scribd.com
Using Spectrophotometry to Determine Concentration(1 Spectrophotometry Absorbance Difference a spectrophotometer measures the amount of light that a sample absorbs. spectrophotometers are instruments designed to detect the amount of light energy that is absorbed or transmitted by molecules dissolved in a solution. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a sample, and can be. in. Spectrophotometry Absorbance Difference.
From www.analyzetest.com
A to Z of UVVis spectroscopy interpretation Spectrophotometry Absorbance Difference A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a sample, and can be. in spectrophotometry, we either measure the absorbance or emission of emr after its interaction with matter. spectrophotometers are instruments designed to detect the amount of light energy that is absorbed or transmitted by molecules dissolved. Spectrophotometry Absorbance Difference.
From shaniyaukung.blogspot.com
What Is Absorbance In Spectrophotometer Spectrophotometry Absorbance Difference spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as. Absorbance is a measure of how much infrared radiation is absorbed by a sample at a particular. in spectrophotometry, we either measure the absorbance or emission of emr after its interaction with matter. A spectrophotometer in an instrument. Spectrophotometry Absorbance Difference.
From namrataheda.blogspot.ca
B for Biology Spectrophotometry UVVisible Spectrophotometry Spectrophotometry Absorbance Difference The instrument operates by passing a beam of. what is the %t for a sample if its absorbance is 1.27? spectrophotometers are instruments designed to detect the amount of light energy that is absorbed or transmitted by molecules dissolved in a solution. Absorbance is a measure of how much infrared radiation is absorbed by a sample at a. Spectrophotometry Absorbance Difference.
From www.numerade.com
SOLVED Describe what is meant by absorbance in spectrophotometry and Spectrophotometry Absorbance Difference a spectrophotometer measures the amount of light that a sample absorbs. Absorbance is a measure of how much infrared radiation is absorbed by a sample at a particular. discover how optical density (od), absorbance, and transmittance interplay to determine concentration in. in spectrophotometry, we either measure the absorbance or emission of emr after its interaction with matter.. Spectrophotometry Absorbance Difference.
From mavink.com
Absorbance Color Wheel Spectrophotometry Absorbance Difference what is the %t for a sample if its absorbance is 1.27? The instrument operates by passing a beam of. a spectrophotometer measures the amount of light that a sample absorbs. discover how optical density (od), absorbance, and transmittance interplay to determine concentration in. Absorbance is a measure of how much infrared radiation is absorbed by a. Spectrophotometry Absorbance Difference.
From pediaa.com
Difference Between Absorbance and Transmittance Spectrophotometry Absorbance Difference a spectrophotometer measures the amount of light that a sample absorbs. discover how optical density (od), absorbance, and transmittance interplay to determine concentration in. spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as. The instrument operates by passing a beam of. spectrophotometers are instruments designed. Spectrophotometry Absorbance Difference.
From quizconsectary.z21.web.core.windows.net
How To Measure Absorbance Spectrophotometry Absorbance Difference what is the %t for a sample if its absorbance is 1.27? spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as. discover how optical density (od), absorbance, and transmittance interplay to determine concentration in. a spectrophotometer measures the amount of light that a sample absorbs.. Spectrophotometry Absorbance Difference.
From chem.libretexts.org
4.12 UVVisible Spectroscopy Chemistry LibreTexts Spectrophotometry Absorbance Difference discover how optical density (od), absorbance, and transmittance interplay to determine concentration in. what is the %t for a sample if its absorbance is 1.27? The instrument operates by passing a beam of. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (λ) by a sample, and can be. spectrophotometry. Spectrophotometry Absorbance Difference.