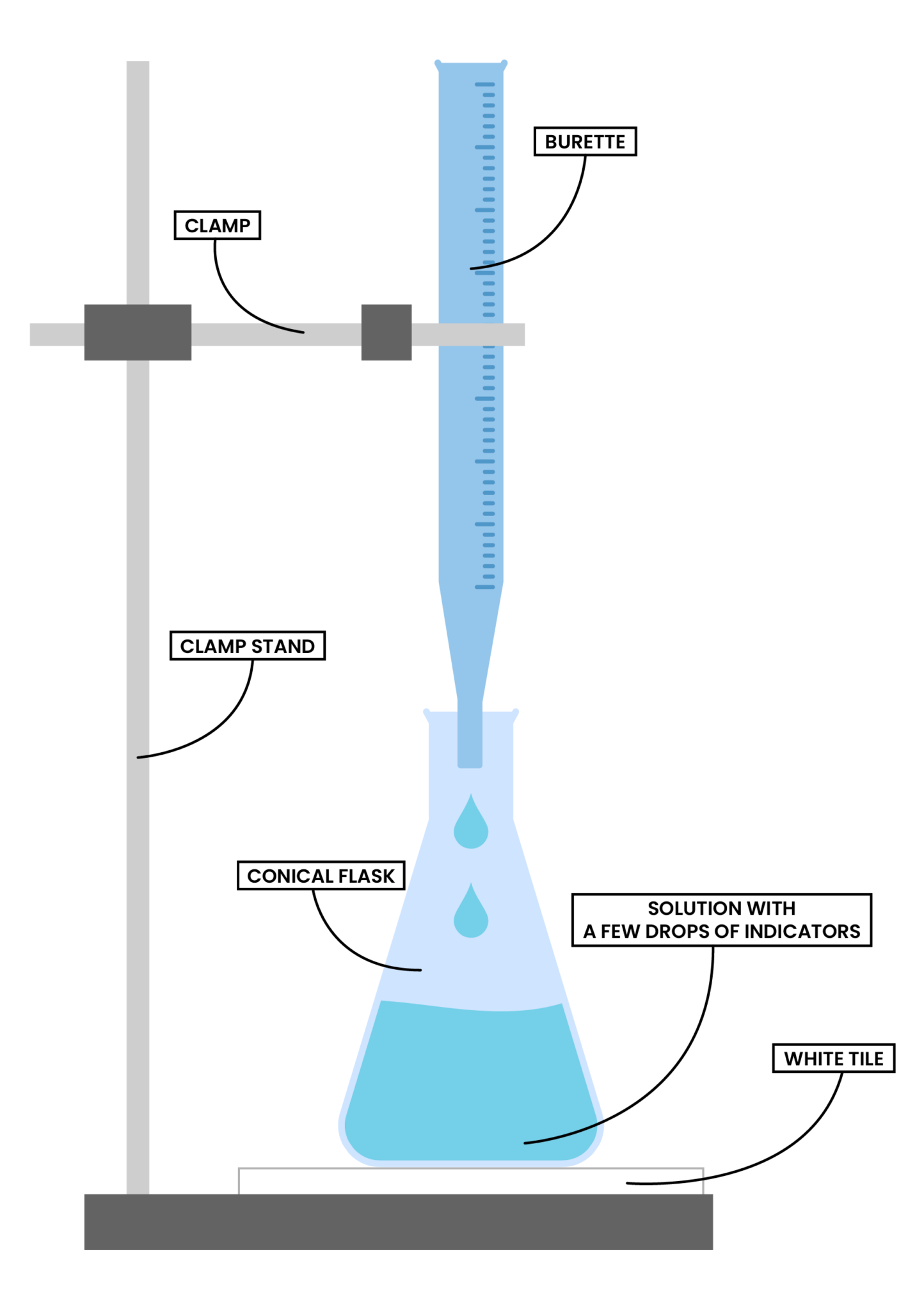
Titration Byju's . The concentration of b can then. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. A known volume of a standard solution (a) is added to a known volume of a solution with unknown concentration (b). Learn the titration graph and titration equation. titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an unknown solution. What is the equivalence point of a titration. Determine the reacting volumes of solutions of acid and alkali by titration and determine the concentration of solutions of acid and alkali by titration. For instance, you might add a standard base solution to an mystery acid solution. What are titrant and analyte. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. a quantitative and volumetric technique, to determine the unknown concentration of a solution by the known concentration of a solution.
from resource.studiaacademy.com
Determine the reacting volumes of solutions of acid and alkali by titration and determine the concentration of solutions of acid and alkali by titration. The concentration of b can then. titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an unknown solution. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. a quantitative and volumetric technique, to determine the unknown concentration of a solution by the known concentration of a solution. What are titrant and analyte. Learn the titration graph and titration equation. A known volume of a standard solution (a) is added to a known volume of a solution with unknown concentration (b). The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. For instance, you might add a standard base solution to an mystery acid solution.
Acids, Alkalis and Titrations Studia Academy Resources
Titration Byju's The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. The concentration of b can then. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. a quantitative and volumetric technique, to determine the unknown concentration of a solution by the known concentration of a solution. For instance, you might add a standard base solution to an mystery acid solution. Learn the titration graph and titration equation. What is the equivalence point of a titration. titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an unknown solution. Determine the reacting volumes of solutions of acid and alkali by titration and determine the concentration of solutions of acid and alkali by titration. A known volume of a standard solution (a) is added to a known volume of a solution with unknown concentration (b). What are titrant and analyte. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent.
From cevfuhpn.blob.core.windows.net
Titration Percentage Formula at Grace Sasso blog Titration Byju's Learn the titration graph and titration equation. Determine the reacting volumes of solutions of acid and alkali by titration and determine the concentration of solutions of acid and alkali by titration. What are titrant and analyte. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. The. Titration Byju's.
From slideplayer.com
Acids and Bases. ppt download Titration Byju's a quantitative and volumetric technique, to determine the unknown concentration of a solution by the known concentration of a solution. A known volume of a standard solution (a) is added to a known volume of a solution with unknown concentration (b). a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using. Titration Byju's.
From www.youtube.com
Difference Btw Conductometric and Potentiometric Titration Concept Titration Byju's What is the equivalence point of a titration. What are titrant and analyte. Learn the titration graph and titration equation. a quantitative and volumetric technique, to determine the unknown concentration of a solution by the known concentration of a solution. titration refers to a process where the use of a solution of known concentration takes place for the. Titration Byju's.
From www.youtube.com
GATE 2025 Civil Engineering Environment Alkalinity Titration Titration Byju's The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. The concentration of b can then. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Learn the titration graph and titration equation. a quantitative. Titration Byju's.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Titration Byju's titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an unknown solution. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. For instance, you might add a standard base solution to. Titration Byju's.
From ck12.org
Titration CK12 Foundation Titration Byju's For instance, you might add a standard base solution to an mystery acid solution. A known volume of a standard solution (a) is added to a known volume of a solution with unknown concentration (b). The concentration of b can then. Determine the reacting volumes of solutions of acid and alkali by titration and determine the concentration of solutions of. Titration Byju's.
From dxovfolwl.blob.core.windows.net
Precipitation Titration Byju's at Jason Hine blog Titration Byju's Determine the reacting volumes of solutions of acid and alkali by titration and determine the concentration of solutions of acid and alkali by titration. What are titrant and analyte. titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an unknown solution. A known volume of. Titration Byju's.
From www.youtube.com
Titrator Auto Titration Equipment YouTube Titration Byju's For instance, you might add a standard base solution to an mystery acid solution. Determine the reacting volumes of solutions of acid and alkali by titration and determine the concentration of solutions of acid and alkali by titration. a quantitative and volumetric technique, to determine the unknown concentration of a solution by the known concentration of a solution. What. Titration Byju's.
From printablenemnserioeg.z22.web.core.windows.net
Titration Practical Questions And Answers Titration Byju's The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an unknown solution. a quantitative and volumetric technique, to determine the unknown. Titration Byju's.
From slideplayer.com
Acids and Bases. ppt download Titration Byju's Learn the titration graph and titration equation. Determine the reacting volumes of solutions of acid and alkali by titration and determine the concentration of solutions of acid and alkali by titration. The concentration of b can then. For instance, you might add a standard base solution to an mystery acid solution. a titration is a laboratory technique used to. Titration Byju's.
From lessonplans.ie
Titrations list Lesson Plans Titration Byju's What is the equivalence point of a titration. a quantitative and volumetric technique, to determine the unknown concentration of a solution by the known concentration of a solution. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. What are titrant and analyte. Determine the reacting. Titration Byju's.
From parholikhotoacknoledge.blogspot.com
Titrations in Chemistry Unveiling the Secrets of Quantitative Analysis Titration Byju's The concentration of b can then. Determine the reacting volumes of solutions of acid and alkali by titration and determine the concentration of solutions of acid and alkali by titration. What is the equivalence point of a titration. a quantitative and volumetric technique, to determine the unknown concentration of a solution by the known concentration of a solution. . Titration Byju's.
From www.chemicals.co.uk
What is Titration Used For in Real Life? The Chemistry Blog Titration Byju's The concentration of b can then. titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an unknown solution. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. What are titrant and analyte. Determine. Titration Byju's.
From www.youtube.com
Advantages of Using Automatic Titration YouTube Titration Byju's What is the equivalence point of a titration. What are titrant and analyte. Determine the reacting volumes of solutions of acid and alkali by titration and determine the concentration of solutions of acid and alkali by titration. The concentration of b can then. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution. Titration Byju's.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts Titration Byju's Determine the reacting volumes of solutions of acid and alkali by titration and determine the concentration of solutions of acid and alkali by titration. a quantitative and volumetric technique, to determine the unknown concentration of a solution by the known concentration of a solution. The concentration of b can then. What are titrant and analyte. A known volume of. Titration Byju's.
From chem.libretexts.org
Acid/Base Titrations Chemistry LibreTexts Titration Byju's titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an unknown solution. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. What is the equivalence point of a titration. Determine the. Titration Byju's.
From www.hindustantimes.com
Byju's, valued at 22 billion in Nov 2022, now at less than 3 billion Titration Byju's a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. For instance, you might add a standard base solution to an mystery acid solution. A known volume of a standard solution (a) is added to a known volume of a solution with unknown concentration (b). titration refers to. Titration Byju's.
From chemistnotes.com
Precipitation Titration Principle, Types, and 5 Reliable Applications Titration Byju's Learn the titration graph and titration equation. What are titrant and analyte. The concentration of b can then. For instance, you might add a standard base solution to an mystery acid solution. A known volume of a standard solution (a) is added to a known volume of a solution with unknown concentration (b). What is the equivalence point of a. Titration Byju's.
From chem-textbook.ucalgary.ca
Finding the Endpoint of a Titration UCalgary Chemistry Textbook Titration Byju's titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an unknown solution. What is the equivalence point of a titration. What are titrant and analyte. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of. Titration Byju's.
From dxovfolwl.blob.core.windows.net
Precipitation Titration Byju's at Jason Hine blog Titration Byju's Learn the titration graph and titration equation. What is the equivalence point of a titration. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A known volume of a standard solution (a) is added to a known volume of a solution with unknown concentration (b). a quantitative. Titration Byju's.
From www.youtube.com
Sodium Thiosulphate Solution preparation and Standardization Titration Byju's For instance, you might add a standard base solution to an mystery acid solution. a quantitative and volumetric technique, to determine the unknown concentration of a solution by the known concentration of a solution. What is the equivalence point of a titration. Determine the reacting volumes of solutions of acid and alkali by titration and determine the concentration of. Titration Byju's.
From mmerevise.co.uk
Titrations and Uncertainties MME Titration Byju's a quantitative and volumetric technique, to determine the unknown concentration of a solution by the known concentration of a solution. A known volume of a standard solution (a) is added to a known volume of a solution with unknown concentration (b). Determine the reacting volumes of solutions of acid and alkali by titration and determine the concentration of solutions. Titration Byju's.
From letitsnowglobe.co.uk
Titration procedure pdf Titration Byju's Learn the titration graph and titration equation. What is the equivalence point of a titration. titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an unknown solution. The basic process involves adding a standard solution of one reagent to a known amount of the unknown. Titration Byju's.
From www.studocu.com
Chapter 14 Acid Base Titration Calculations in the context of Titration Byju's What are titrant and analyte. The concentration of b can then. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. What is the equivalence point of a titration. A known volume of a standard solution (a) is added to a known volume of a solution with unknown concentration. Titration Byju's.
From dxovfolwl.blob.core.windows.net
Precipitation Titration Byju's at Jason Hine blog Titration Byju's For instance, you might add a standard base solution to an mystery acid solution. A known volume of a standard solution (a) is added to a known volume of a solution with unknown concentration (b). a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Determine the reacting volumes. Titration Byju's.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Byju's a quantitative and volumetric technique, to determine the unknown concentration of a solution by the known concentration of a solution. Learn the titration graph and titration equation. A known volume of a standard solution (a) is added to a known volume of a solution with unknown concentration (b). a titration is a laboratory technique used to precisely measure. Titration Byju's.
From dxovfolwl.blob.core.windows.net
Precipitation Titration Byju's at Jason Hine blog Titration Byju's Determine the reacting volumes of solutions of acid and alkali by titration and determine the concentration of solutions of acid and alkali by titration. A known volume of a standard solution (a) is added to a known volume of a solution with unknown concentration (b). Learn the titration graph and titration equation. What are titrant and analyte. The concentration of. Titration Byju's.
From mmerevise.co.uk
Titrations and Uncertainties MME Titration Byju's Learn the titration graph and titration equation. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. A known volume of a standard solution (a) is added to a known volume of a solution with unknown concentration (b). a titration is a laboratory technique used to. Titration Byju's.
From www.youtube.com
Titration Calculations Exam Style Questions Revision for ALevel Titration Byju's What is the equivalence point of a titration. Learn the titration graph and titration equation. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. For instance, you might add a standard base solution to an mystery acid solution. A known volume of a standard solution (a) is added. Titration Byju's.
From www.aiophotoz.com
What Is Titration And How Is It Done Chemistry Made Simple Images and Titration Byju's Learn the titration graph and titration equation. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A known volume of a standard solution (a) is added to a known volume of a solution with unknown concentration (b). For instance, you might add a standard base solution to an. Titration Byju's.
From resource.studiaacademy.com
Acids, Alkalis and Titrations Studia Academy Resources Titration Byju's a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A known volume of a standard solution (a) is added to a known volume of a solution with unknown concentration (b). Determine the reacting volumes of solutions of acid and alkali by titration and determine the concentration of solutions. Titration Byju's.
From chem4three.blogspot.ca
CHEMISTRY 11 TITRATIONS Titration Byju's a quantitative and volumetric technique, to determine the unknown concentration of a solution by the known concentration of a solution. For instance, you might add a standard base solution to an mystery acid solution. titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an. Titration Byju's.
From www.chemicals.co.uk
What is Titration in Chemistry? The Chemistry Blog Titration Byju's The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. Determine the reacting volumes of solutions of acid and alkali by titration and determine the concentration of solutions of acid and alkali by titration. titration refers to a process where the use of a solution of. Titration Byju's.
From moodle.tau.ac.il
AcidBase Titration Curves Titration Byju's The concentration of b can then. What is the equivalence point of a titration. What are titrant and analyte. a quantitative and volumetric technique, to determine the unknown concentration of a solution by the known concentration of a solution. Learn the titration graph and titration equation. a titration is a laboratory technique used to precisely measure molar concentration. Titration Byju's.
From www.scribd.com
Titration Curves (Autosaved) PDF Titration Chemistry Titration Byju's a quantitative and volumetric technique, to determine the unknown concentration of a solution by the known concentration of a solution. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Determine the reacting volumes of solutions of acid and alkali by titration and determine the concentration of solutions. Titration Byju's.