Medical Device Class India . ministry of health & family welfare, government of india has released the medical device rules, 2017, effective. the central drugs standard control organization (cdsco) under the ministry of health & family welfare. medical device is safe and performs as intended by the manufacturer and therefore conforms to the essential principles of. he central drugs standard control organisation(cdsco)under directorate general of health services,ministry of. 89 rows medical devices are classified into four class based on the risk parameter as specified in part i of the first schedule of. rdable, and comprehensive healthcare to all citizens. based on intended use of the device, risk associated with the device and other parameters referred to in imdr, the.
from www.thermofisher.cn
medical device is safe and performs as intended by the manufacturer and therefore conforms to the essential principles of. 89 rows medical devices are classified into four class based on the risk parameter as specified in part i of the first schedule of. ministry of health & family welfare, government of india has released the medical device rules, 2017, effective. based on intended use of the device, risk associated with the device and other parameters referred to in imdr, the. rdable, and comprehensive healthcare to all citizens. the central drugs standard control organization (cdsco) under the ministry of health & family welfare. he central drugs standard control organisation(cdsco)under directorate general of health services,ministry of.
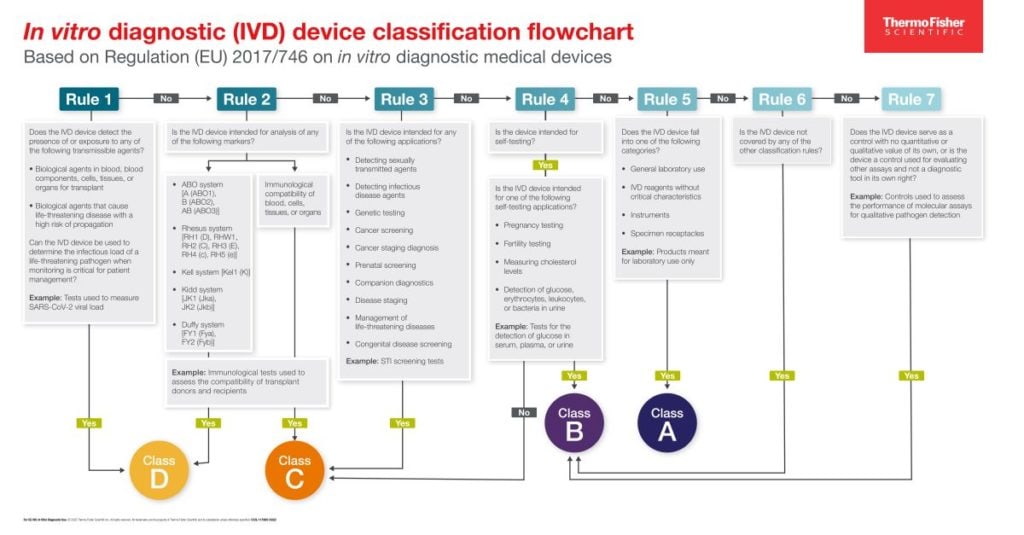
IVDD vs. IVDR Classifications Defined and Compared OEMpowered
Medical Device Class India based on intended use of the device, risk associated with the device and other parameters referred to in imdr, the. ministry of health & family welfare, government of india has released the medical device rules, 2017, effective. based on intended use of the device, risk associated with the device and other parameters referred to in imdr, the. rdable, and comprehensive healthcare to all citizens. 89 rows medical devices are classified into four class based on the risk parameter as specified in part i of the first schedule of. medical device is safe and performs as intended by the manufacturer and therefore conforms to the essential principles of. the central drugs standard control organization (cdsco) under the ministry of health & family welfare. he central drugs standard control organisation(cdsco)under directorate general of health services,ministry of.
From www.sycaimedical.com
Overview on the regulatory path for software medical devices Medical Device Class India rdable, and comprehensive healthcare to all citizens. ministry of health & family welfare, government of india has released the medical device rules, 2017, effective. medical device is safe and performs as intended by the manufacturer and therefore conforms to the essential principles of. 89 rows medical devices are classified into four class based on the risk. Medical Device Class India.
From www.vrogue.co
The 3 Fda Medical Device Classes Differences And Exam vrogue.co Medical Device Class India rdable, and comprehensive healthcare to all citizens. the central drugs standard control organization (cdsco) under the ministry of health & family welfare. based on intended use of the device, risk associated with the device and other parameters referred to in imdr, the. 89 rows medical devices are classified into four class based on the risk parameter. Medical Device Class India.
From laegemiddelstyrelsen.dk
Medical devices Medical Device Class India ministry of health & family welfare, government of india has released the medical device rules, 2017, effective. the central drugs standard control organization (cdsco) under the ministry of health & family welfare. 89 rows medical devices are classified into four class based on the risk parameter as specified in part i of the first schedule of. . Medical Device Class India.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Medical Device Class India he central drugs standard control organisation(cdsco)under directorate general of health services,ministry of. based on intended use of the device, risk associated with the device and other parameters referred to in imdr, the. 89 rows medical devices are classified into four class based on the risk parameter as specified in part i of the first schedule of. . Medical Device Class India.
From medicaldevicehq.com
Different classifications rules for medical device software An Medical Device Class India ministry of health & family welfare, government of india has released the medical device rules, 2017, effective. based on intended use of the device, risk associated with the device and other parameters referred to in imdr, the. the central drugs standard control organization (cdsco) under the ministry of health & family welfare. 89 rows medical devices. Medical Device Class India.
From www.rimsys.io
FDA Class II medical devices Medical Device Class India 89 rows medical devices are classified into four class based on the risk parameter as specified in part i of the first schedule of. ministry of health & family welfare, government of india has released the medical device rules, 2017, effective. medical device is safe and performs as intended by the manufacturer and therefore conforms to the. Medical Device Class India.
From www.vrogue.co
Device Classification In India Infographic vrogue.co Medical Device Class India ministry of health & family welfare, government of india has released the medical device rules, 2017, effective. medical device is safe and performs as intended by the manufacturer and therefore conforms to the essential principles of. the central drugs standard control organization (cdsco) under the ministry of health & family welfare. based on intended use of. Medical Device Class India.
From turacoz.com
Regulations of Medical Devices in India An Update Turacoz Healthcare Medical Device Class India medical device is safe and performs as intended by the manufacturer and therefore conforms to the essential principles of. ministry of health & family welfare, government of india has released the medical device rules, 2017, effective. he central drugs standard control organisation(cdsco)under directorate general of health services,ministry of. 89 rows medical devices are classified into four. Medical Device Class India.
From www.qualio.com
The 3 FDA medical device classes differences and examples explained Medical Device Class India he central drugs standard control organisation(cdsco)under directorate general of health services,ministry of. the central drugs standard control organization (cdsco) under the ministry of health & family welfare. ministry of health & family welfare, government of india has released the medical device rules, 2017, effective. 89 rows medical devices are classified into four class based on the. Medical Device Class India.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Medical Device Class India ministry of health & family welfare, government of india has released the medical device rules, 2017, effective. the central drugs standard control organization (cdsco) under the ministry of health & family welfare. 89 rows medical devices are classified into four class based on the risk parameter as specified in part i of the first schedule of. . Medical Device Class India.
From www.pinterest.com
Infographic on Understanding FDA Device Classes from Medical Device Class India based on intended use of the device, risk associated with the device and other parameters referred to in imdr, the. the central drugs standard control organization (cdsco) under the ministry of health & family welfare. ministry of health & family welfare, government of india has released the medical device rules, 2017, effective. rdable, and comprehensive healthcare. Medical Device Class India.
From royed.in
1 Week Competency Development Program Royed Training Medical Device Class India rdable, and comprehensive healthcare to all citizens. based on intended use of the device, risk associated with the device and other parameters referred to in imdr, the. medical device is safe and performs as intended by the manufacturer and therefore conforms to the essential principles of. 89 rows medical devices are classified into four class based. Medical Device Class India.
From www.pacificbridgemedical.com
Device Classification in India Infographic Medical Device Class India based on intended use of the device, risk associated with the device and other parameters referred to in imdr, the. ministry of health & family welfare, government of india has released the medical device rules, 2017, effective. medical device is safe and performs as intended by the manufacturer and therefore conforms to the essential principles of. . Medical Device Class India.
From www.researchgate.net
Medical Device Classification System Download Table Medical Device Class India he central drugs standard control organisation(cdsco)under directorate general of health services,ministry of. rdable, and comprehensive healthcare to all citizens. the central drugs standard control organization (cdsco) under the ministry of health & family welfare. medical device is safe and performs as intended by the manufacturer and therefore conforms to the essential principles of. ministry of. Medical Device Class India.
From exovighly.blob.core.windows.net
Fda Medical Device Private Label Distributor at Jerry Robertson blog Medical Device Class India ministry of health & family welfare, government of india has released the medical device rules, 2017, effective. rdable, and comprehensive healthcare to all citizens. medical device is safe and performs as intended by the manufacturer and therefore conforms to the essential principles of. he central drugs standard control organisation(cdsco)under directorate general of health services,ministry of. . Medical Device Class India.
From www.volersystems.com
Are You Making a Medical Device? Voler Systems Medical Device Class India medical device is safe and performs as intended by the manufacturer and therefore conforms to the essential principles of. he central drugs standard control organisation(cdsco)under directorate general of health services,ministry of. the central drugs standard control organization (cdsco) under the ministry of health & family welfare. rdable, and comprehensive healthcare to all citizens. ministry of. Medical Device Class India.
From mungfali.com
Classification Of Medical Devices Medical Device Class India 89 rows medical devices are classified into four class based on the risk parameter as specified in part i of the first schedule of. the central drugs standard control organization (cdsco) under the ministry of health & family welfare. based on intended use of the device, risk associated with the device and other parameters referred to in. Medical Device Class India.
From www.youtube.com
Medical Device Classes YouTube Medical Device Class India ministry of health & family welfare, government of india has released the medical device rules, 2017, effective. the central drugs standard control organization (cdsco) under the ministry of health & family welfare. medical device is safe and performs as intended by the manufacturer and therefore conforms to the essential principles of. rdable, and comprehensive healthcare to. Medical Device Class India.
From learn.marsdd.com
Medical device submissions Placing a medical device on the market Medical Device Class India ministry of health & family welfare, government of india has released the medical device rules, 2017, effective. he central drugs standard control organisation(cdsco)under directorate general of health services,ministry of. the central drugs standard control organization (cdsco) under the ministry of health & family welfare. 89 rows medical devices are classified into four class based on the. Medical Device Class India.
From exowmprwm.blob.core.windows.net
Medical Device Classes Examples at Thad Bracamonte blog Medical Device Class India medical device is safe and performs as intended by the manufacturer and therefore conforms to the essential principles of. rdable, and comprehensive healthcare to all citizens. the central drugs standard control organization (cdsco) under the ministry of health & family welfare. he central drugs standard control organisation(cdsco)under directorate general of health services,ministry of. based on. Medical Device Class India.
From laegemiddelstyrelsen.dk
Development of medical devices Medical Device Class India he central drugs standard control organisation(cdsco)under directorate general of health services,ministry of. rdable, and comprehensive healthcare to all citizens. the central drugs standard control organization (cdsco) under the ministry of health & family welfare. 89 rows medical devices are classified into four class based on the risk parameter as specified in part i of the first. Medical Device Class India.
From www.thermofisher.cn
IVDD vs. IVDR Classifications Defined and Compared OEMpowered Medical Device Class India ministry of health & family welfare, government of india has released the medical device rules, 2017, effective. medical device is safe and performs as intended by the manufacturer and therefore conforms to the essential principles of. rdable, and comprehensive healthcare to all citizens. based on intended use of the device, risk associated with the device and. Medical Device Class India.
From exowmprwm.blob.core.windows.net
Medical Device Classes Examples at Thad Bracamonte blog Medical Device Class India medical device is safe and performs as intended by the manufacturer and therefore conforms to the essential principles of. the central drugs standard control organization (cdsco) under the ministry of health & family welfare. rdable, and comprehensive healthcare to all citizens. ministry of health & family welfare, government of india has released the medical device rules,. Medical Device Class India.
From chinameddevice.com
CFDA New Medical Device Classification Catalogue Effective August 1st Medical Device Class India ministry of health & family welfare, government of india has released the medical device rules, 2017, effective. 89 rows medical devices are classified into four class based on the risk parameter as specified in part i of the first schedule of. based on intended use of the device, risk associated with the device and other parameters referred. Medical Device Class India.
From www.eos-intelligence.com
Indian Medical Device Rules a Step towards a Better Future Medical Device Class India 89 rows medical devices are classified into four class based on the risk parameter as specified in part i of the first schedule of. rdable, and comprehensive healthcare to all citizens. the central drugs standard control organization (cdsco) under the ministry of health & family welfare. medical device is safe and performs as intended by the. Medical Device Class India.
From issuu.com
Registration Process for Class A NS/NM Medical Devices in India RSI Medical Device Class India the central drugs standard control organization (cdsco) under the ministry of health & family welfare. ministry of health & family welfare, government of india has released the medical device rules, 2017, effective. medical device is safe and performs as intended by the manufacturer and therefore conforms to the essential principles of. based on intended use of. Medical Device Class India.
From www.arenasolutions.com
How to Classify Your Medical Device for FDA Approval Arena Medical Device Class India he central drugs standard control organisation(cdsco)under directorate general of health services,ministry of. ministry of health & family welfare, government of india has released the medical device rules, 2017, effective. 89 rows medical devices are classified into four class based on the risk parameter as specified in part i of the first schedule of. the central drugs. Medical Device Class India.
From www.cognidox.com
New IVD regulation is coming. are you ready? Medical Device Class India he central drugs standard control organisation(cdsco)under directorate general of health services,ministry of. rdable, and comprehensive healthcare to all citizens. 89 rows medical devices are classified into four class based on the risk parameter as specified in part i of the first schedule of. based on intended use of the device, risk associated with the device and. Medical Device Class India.
From www.youtube.com
Medical Device Classes Explained A Beginner's Guide YouTube Medical Device Class India ministry of health & family welfare, government of india has released the medical device rules, 2017, effective. medical device is safe and performs as intended by the manufacturer and therefore conforms to the essential principles of. rdable, and comprehensive healthcare to all citizens. 89 rows medical devices are classified into four class based on the risk. Medical Device Class India.
From angelanjohnson.com
Medical Devices Angela N Johnson Medical Device Class India the central drugs standard control organization (cdsco) under the ministry of health & family welfare. 89 rows medical devices are classified into four class based on the risk parameter as specified in part i of the first schedule of. he central drugs standard control organisation(cdsco)under directorate general of health services,ministry of. ministry of health & family. Medical Device Class India.
From gbu-taganskij.ru
Medical Device Classification According To The MDR Complete, 56 OFF Medical Device Class India 89 rows medical devices are classified into four class based on the risk parameter as specified in part i of the first schedule of. ministry of health & family welfare, government of india has released the medical device rules, 2017, effective. the central drugs standard control organization (cdsco) under the ministry of health & family welfare. . Medical Device Class India.
From www.vrogue.co
The 3 Fda Medical Device Classes Differences And Exam vrogue.co Medical Device Class India medical device is safe and performs as intended by the manufacturer and therefore conforms to the essential principles of. the central drugs standard control organization (cdsco) under the ministry of health & family welfare. ministry of health & family welfare, government of india has released the medical device rules, 2017, effective. based on intended use of. Medical Device Class India.
From in.pinterest.com
India's Medical Devices Sector Medical, Medical device, Marketing Medical Device Class India he central drugs standard control organisation(cdsco)under directorate general of health services,ministry of. 89 rows medical devices are classified into four class based on the risk parameter as specified in part i of the first schedule of. medical device is safe and performs as intended by the manufacturer and therefore conforms to the essential principles of. rdable,. Medical Device Class India.
From www.infoholicresearch.com
Classification Of Newly Notified Medical Devices In India Infoholic Medical Device Class India ministry of health & family welfare, government of india has released the medical device rules, 2017, effective. he central drugs standard control organisation(cdsco)under directorate general of health services,ministry of. based on intended use of the device, risk associated with the device and other parameters referred to in imdr, the. 89 rows medical devices are classified into. Medical Device Class India.
From www.vrogue.co
The 3 Fda Medical Device Classes Differences And Exam vrogue.co Medical Device Class India medical device is safe and performs as intended by the manufacturer and therefore conforms to the essential principles of. ministry of health & family welfare, government of india has released the medical device rules, 2017, effective. rdable, and comprehensive healthcare to all citizens. the central drugs standard control organization (cdsco) under the ministry of health &. Medical Device Class India.