What Temperature Does Water Evaporate Under Vacuum . when liquid water meets dry air, it is not in equilibrium; This is why if you go to a high altitude location (like. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour. online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and imperial units. The boiling point at different levels of vacuum can be. water actually boils at a lower temperature if the pressure around it is lowered. water boiling temperature vs pressure in vacuum table chart. this effectively increases the boiling point of the liquid and it takes a higher temperature to evaporate all the liquid (e.g. The boiling point of water is the temperature at which the. water boils when the vapor pressure is greater than the ambient pressure. 95 rows when water is heated up under vacuum, its boiling point is lower than at atmospheric pressure.
from vt.audubon.org
This is why if you go to a high altitude location (like. The boiling point at different levels of vacuum can be. water boils when the vapor pressure is greater than the ambient pressure. The boiling point of water is the temperature at which the. this effectively increases the boiling point of the liquid and it takes a higher temperature to evaporate all the liquid (e.g. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour. 95 rows when water is heated up under vacuum, its boiling point is lower than at atmospheric pressure. water actually boils at a lower temperature if the pressure around it is lowered. water boiling temperature vs pressure in vacuum table chart. when liquid water meets dry air, it is not in equilibrium;
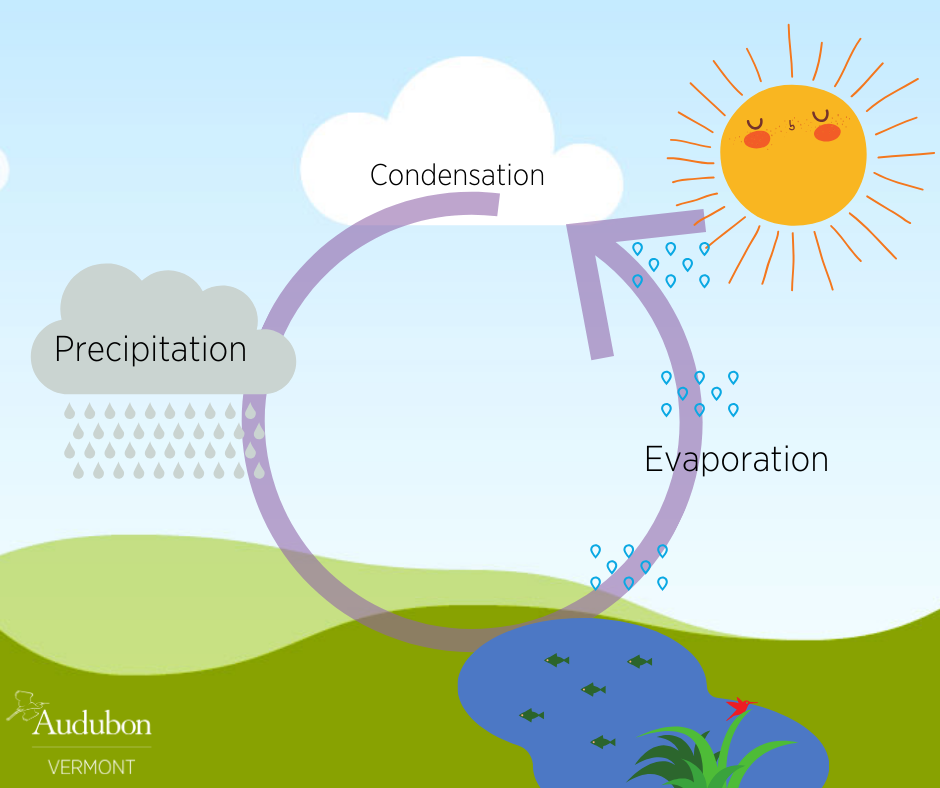
The Water Cycle Revisited! Audubon Vermont
What Temperature Does Water Evaporate Under Vacuum This is why if you go to a high altitude location (like. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour. online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and imperial units. water actually boils at a lower temperature if the pressure around it is lowered. water boiling temperature vs pressure in vacuum table chart. 95 rows when water is heated up under vacuum, its boiling point is lower than at atmospheric pressure. The boiling point of water is the temperature at which the. This is why if you go to a high altitude location (like. The boiling point at different levels of vacuum can be. this effectively increases the boiling point of the liquid and it takes a higher temperature to evaporate all the liquid (e.g. when liquid water meets dry air, it is not in equilibrium; water boils when the vapor pressure is greater than the ambient pressure.
From watermarkexplorer.blogspot.com
Evaporation Water Evaporation Temperature What Temperature Does Water Evaporate Under Vacuum This is why if you go to a high altitude location (like. The boiling point at different levels of vacuum can be. water boiling temperature vs pressure in vacuum table chart. The boiling point of water is the temperature at which the. this effectively increases the boiling point of the liquid and it takes a higher temperature to. What Temperature Does Water Evaporate Under Vacuum.
From www.youtube.com
Vacuum pump boiling water at room temperature YouTube What Temperature Does Water Evaporate Under Vacuum The boiling point of water is the temperature at which the. this effectively increases the boiling point of the liquid and it takes a higher temperature to evaporate all the liquid (e.g. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour. This is why if you go to a high altitude. What Temperature Does Water Evaporate Under Vacuum.
From sciencenotes.org
How to Boil Water at Room Temperature What Temperature Does Water Evaporate Under Vacuum water actually boils at a lower temperature if the pressure around it is lowered. water boils when the vapor pressure is greater than the ambient pressure. This is why if you go to a high altitude location (like. The boiling point of water is the temperature at which the. The boiling point at different levels of vacuum can. What Temperature Does Water Evaporate Under Vacuum.
From mavink.com
Water Evaporation Chart What Temperature Does Water Evaporate Under Vacuum Water molecules evaporate off the surface until the amount of water in the air creates enough vapour. water actually boils at a lower temperature if the pressure around it is lowered. 95 rows when water is heated up under vacuum, its boiling point is lower than at atmospheric pressure. The boiling point of water is the temperature at. What Temperature Does Water Evaporate Under Vacuum.
From www.youtube.com
Evaporation, Vapor Pressure and Boiling YouTube What Temperature Does Water Evaporate Under Vacuum water boiling temperature vs pressure in vacuum table chart. This is why if you go to a high altitude location (like. 95 rows when water is heated up under vacuum, its boiling point is lower than at atmospheric pressure. water boils when the vapor pressure is greater than the ambient pressure. Water molecules evaporate off the surface. What Temperature Does Water Evaporate Under Vacuum.
From sarvowater.com
Mechanical Vapor Sarvo Water What Temperature Does Water Evaporate Under Vacuum online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and imperial units. The boiling point at different levels of vacuum can be. The boiling point of water is the temperature at which the. water actually boils at a lower temperature if the pressure around it is lowered. Water molecules evaporate off the. What Temperature Does Water Evaporate Under Vacuum.
From saltworkconsultants.com
Physics of evaporation What Temperature Does Water Evaporate Under Vacuum The boiling point at different levels of vacuum can be. water actually boils at a lower temperature if the pressure around it is lowered. 95 rows when water is heated up under vacuum, its boiling point is lower than at atmospheric pressure. this effectively increases the boiling point of the liquid and it takes a higher temperature. What Temperature Does Water Evaporate Under Vacuum.
From www.scienceabc.com
Why Does Water Evaporate At Room Temperature? What Temperature Does Water Evaporate Under Vacuum The boiling point of water is the temperature at which the. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour. The boiling point at different levels of vacuum can be. water actually boils at a lower temperature if the pressure around it is lowered. 95 rows when water is heated. What Temperature Does Water Evaporate Under Vacuum.
From chemistry.stackexchange.com
solutions why do we obtain a sigmoid curve in vapour pressure versus What Temperature Does Water Evaporate Under Vacuum 95 rows when water is heated up under vacuum, its boiling point is lower than at atmospheric pressure. The boiling point at different levels of vacuum can be. water actually boils at a lower temperature if the pressure around it is lowered. Water molecules evaporate off the surface until the amount of water in the air creates enough. What Temperature Does Water Evaporate Under Vacuum.
From vt.audubon.org
The Water Cycle Revisited! Audubon Vermont What Temperature Does Water Evaporate Under Vacuum this effectively increases the boiling point of the liquid and it takes a higher temperature to evaporate all the liquid (e.g. when liquid water meets dry air, it is not in equilibrium; This is why if you go to a high altitude location (like. water actually boils at a lower temperature if the pressure around it is. What Temperature Does Water Evaporate Under Vacuum.
From www.chemicals.co.uk
What is the Definition of Evaporation in Chemistry? What Temperature Does Water Evaporate Under Vacuum The boiling point at different levels of vacuum can be. this effectively increases the boiling point of the liquid and it takes a higher temperature to evaporate all the liquid (e.g. 95 rows when water is heated up under vacuum, its boiling point is lower than at atmospheric pressure. water actually boils at a lower temperature if. What Temperature Does Water Evaporate Under Vacuum.
From www.scienceabc.com
Why Does Water Evaporate At Room Temperature? What Temperature Does Water Evaporate Under Vacuum The boiling point of water is the temperature at which the. water actually boils at a lower temperature if the pressure around it is lowered. 95 rows when water is heated up under vacuum, its boiling point is lower than at atmospheric pressure. online calculator, figures and tables giving the boiling temperatures of water in varying vacuum,. What Temperature Does Water Evaporate Under Vacuum.
From puretechenvironmental.com
Evaporation Puretech Environmental What Temperature Does Water Evaporate Under Vacuum Water molecules evaporate off the surface until the amount of water in the air creates enough vapour. The boiling point at different levels of vacuum can be. This is why if you go to a high altitude location (like. when liquid water meets dry air, it is not in equilibrium; this effectively increases the boiling point of the. What Temperature Does Water Evaporate Under Vacuum.
From www.youtube.com
How does water evaporate at room temperaturevaporization and What Temperature Does Water Evaporate Under Vacuum water boils when the vapor pressure is greater than the ambient pressure. The boiling point at different levels of vacuum can be. water actually boils at a lower temperature if the pressure around it is lowered. This is why if you go to a high altitude location (like. Water molecules evaporate off the surface until the amount of. What Temperature Does Water Evaporate Under Vacuum.
From dxoetofzk.blob.core.windows.net
Evaporation Under Vacuum at Daniel Orton blog What Temperature Does Water Evaporate Under Vacuum water boils when the vapor pressure is greater than the ambient pressure. The boiling point at different levels of vacuum can be. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour. when liquid water meets dry air, it is not in equilibrium; This is why if you go to a. What Temperature Does Water Evaporate Under Vacuum.
From dxowlbddi.blob.core.windows.net
Explain The Evaporation Process at James Ligon blog What Temperature Does Water Evaporate Under Vacuum This is why if you go to a high altitude location (like. The boiling point of water is the temperature at which the. online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and imperial units. this effectively increases the boiling point of the liquid and it takes a higher temperature to evaporate. What Temperature Does Water Evaporate Under Vacuum.
From mavink.com
Vaporizer Temperature Chart What Temperature Does Water Evaporate Under Vacuum The boiling point at different levels of vacuum can be. when liquid water meets dry air, it is not in equilibrium; The boiling point of water is the temperature at which the. water boils when the vapor pressure is greater than the ambient pressure. Water molecules evaporate off the surface until the amount of water in the air. What Temperature Does Water Evaporate Under Vacuum.
From philschatz.com
Phase Change and Latent Heat · Physics What Temperature Does Water Evaporate Under Vacuum 95 rows when water is heated up under vacuum, its boiling point is lower than at atmospheric pressure. water boils when the vapor pressure is greater than the ambient pressure. The boiling point at different levels of vacuum can be. online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and imperial. What Temperature Does Water Evaporate Under Vacuum.
From young6science3.weebly.com
5Th grade science The Water Cycle What Temperature Does Water Evaporate Under Vacuum online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and imperial units. when liquid water meets dry air, it is not in equilibrium; The boiling point at different levels of vacuum can be. This is why if you go to a high altitude location (like. this effectively increases the boiling point. What Temperature Does Water Evaporate Under Vacuum.
From slideplayer.com
Phase Changes and Intermolecular Forces ppt download What Temperature Does Water Evaporate Under Vacuum This is why if you go to a high altitude location (like. online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and imperial units. water actually boils at a lower temperature if the pressure around it is lowered. water boiling temperature vs pressure in vacuum table chart. water boils when. What Temperature Does Water Evaporate Under Vacuum.
From 9to5science.com
[Solved] Why does water evaporate at room temperature? 9to5Science What Temperature Does Water Evaporate Under Vacuum Water molecules evaporate off the surface until the amount of water in the air creates enough vapour. 95 rows when water is heated up under vacuum, its boiling point is lower than at atmospheric pressure. online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and imperial units. The boiling point of water. What Temperature Does Water Evaporate Under Vacuum.
From gfecc.org
Gallery of liquid ring vacuum pump working principle and pumping system What Temperature Does Water Evaporate Under Vacuum this effectively increases the boiling point of the liquid and it takes a higher temperature to evaporate all the liquid (e.g. This is why if you go to a high altitude location (like. water boiling temperature vs pressure in vacuum table chart. 95 rows when water is heated up under vacuum, its boiling point is lower than. What Temperature Does Water Evaporate Under Vacuum.
From www.researchgate.net
Amount of heat needed to vaporize water having initial temperatures of What Temperature Does Water Evaporate Under Vacuum The boiling point at different levels of vacuum can be. The boiling point of water is the temperature at which the. This is why if you go to a high altitude location (like. water actually boils at a lower temperature if the pressure around it is lowered. 95 rows when water is heated up under vacuum, its boiling. What Temperature Does Water Evaporate Under Vacuum.
From www.researchgate.net
Evolution of water vapor pressure Pvapor along with interfacial What Temperature Does Water Evaporate Under Vacuum water actually boils at a lower temperature if the pressure around it is lowered. This is why if you go to a high altitude location (like. online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and imperial units. water boiling temperature vs pressure in vacuum table chart. 95 rows when. What Temperature Does Water Evaporate Under Vacuum.
From vacaero.com
Simple Physics for the High Vacuum Processing Industry What Temperature Does Water Evaporate Under Vacuum The boiling point at different levels of vacuum can be. 95 rows when water is heated up under vacuum, its boiling point is lower than at atmospheric pressure. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour. This is why if you go to a high altitude location (like. when. What Temperature Does Water Evaporate Under Vacuum.
From diagramlibraryflew.z13.web.core.windows.net
Diagram Of Evaporation What Temperature Does Water Evaporate Under Vacuum The boiling point of water is the temperature at which the. when liquid water meets dry air, it is not in equilibrium; Water molecules evaporate off the surface until the amount of water in the air creates enough vapour. The boiling point at different levels of vacuum can be. this effectively increases the boiling point of the liquid. What Temperature Does Water Evaporate Under Vacuum.
From scindustrialsales.com
Boiling Point of Water Under Vacuum Pumps and Filtration What Temperature Does Water Evaporate Under Vacuum The boiling point at different levels of vacuum can be. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour. The boiling point of water is the temperature at which the. this effectively increases the boiling point of the liquid and it takes a higher temperature to evaporate all the liquid (e.g.. What Temperature Does Water Evaporate Under Vacuum.
From basc.pnnl.gov
Evaporative Cooling Systems Building America Solution Center What Temperature Does Water Evaporate Under Vacuum 95 rows when water is heated up under vacuum, its boiling point is lower than at atmospheric pressure. when liquid water meets dry air, it is not in equilibrium; This is why if you go to a high altitude location (like. The boiling point of water is the temperature at which the. water actually boils at a. What Temperature Does Water Evaporate Under Vacuum.
From monica-well-sims.blogspot.com
A Substance That Evaporates at Room Temperature Is Described as What Temperature Does Water Evaporate Under Vacuum 95 rows when water is heated up under vacuum, its boiling point is lower than at atmospheric pressure. when liquid water meets dry air, it is not in equilibrium; water boils when the vapor pressure is greater than the ambient pressure. The boiling point of water is the temperature at which the. Water molecules evaporate off the. What Temperature Does Water Evaporate Under Vacuum.
From www.snexplores.org
Explainer What are the different states of matter? What Temperature Does Water Evaporate Under Vacuum water boiling temperature vs pressure in vacuum table chart. The boiling point of water is the temperature at which the. online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and imperial units. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour. this effectively. What Temperature Does Water Evaporate Under Vacuum.
From learningzonegregorin2m.z4.web.core.windows.net
Heat Of Vaporization Pdf What Temperature Does Water Evaporate Under Vacuum online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and imperial units. This is why if you go to a high altitude location (like. The boiling point at different levels of vacuum can be. water boiling temperature vs pressure in vacuum table chart. when liquid water meets dry air, it is. What Temperature Does Water Evaporate Under Vacuum.
From physics.stackexchange.com
thermodynamics Evaporation of water at room temperature Physics What Temperature Does Water Evaporate Under Vacuum The boiling point at different levels of vacuum can be. water actually boils at a lower temperature if the pressure around it is lowered. 95 rows when water is heated up under vacuum, its boiling point is lower than at atmospheric pressure. The boiling point of water is the temperature at which the. water boiling temperature vs. What Temperature Does Water Evaporate Under Vacuum.
From www.metoffice.gov.uk
The water cycle Met Office What Temperature Does Water Evaporate Under Vacuum 95 rows when water is heated up under vacuum, its boiling point is lower than at atmospheric pressure. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour. water actually boils at a lower temperature if the pressure around it is lowered. when liquid water meets dry air, it is. What Temperature Does Water Evaporate Under Vacuum.
From learncheme.com
evaporativecoolingofwater LearnChemE What Temperature Does Water Evaporate Under Vacuum this effectively increases the boiling point of the liquid and it takes a higher temperature to evaporate all the liquid (e.g. 95 rows when water is heated up under vacuum, its boiling point is lower than at atmospheric pressure. water boils when the vapor pressure is greater than the ambient pressure. water actually boils at a. What Temperature Does Water Evaporate Under Vacuum.
From www.ck12.org
Heating and Cooling Curves ( Read ) Chemistry CK12 Foundation What Temperature Does Water Evaporate Under Vacuum online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and imperial units. this effectively increases the boiling point of the liquid and it takes a higher temperature to evaporate all the liquid (e.g. water boils when the vapor pressure is greater than the ambient pressure. The boiling point of water is. What Temperature Does Water Evaporate Under Vacuum.