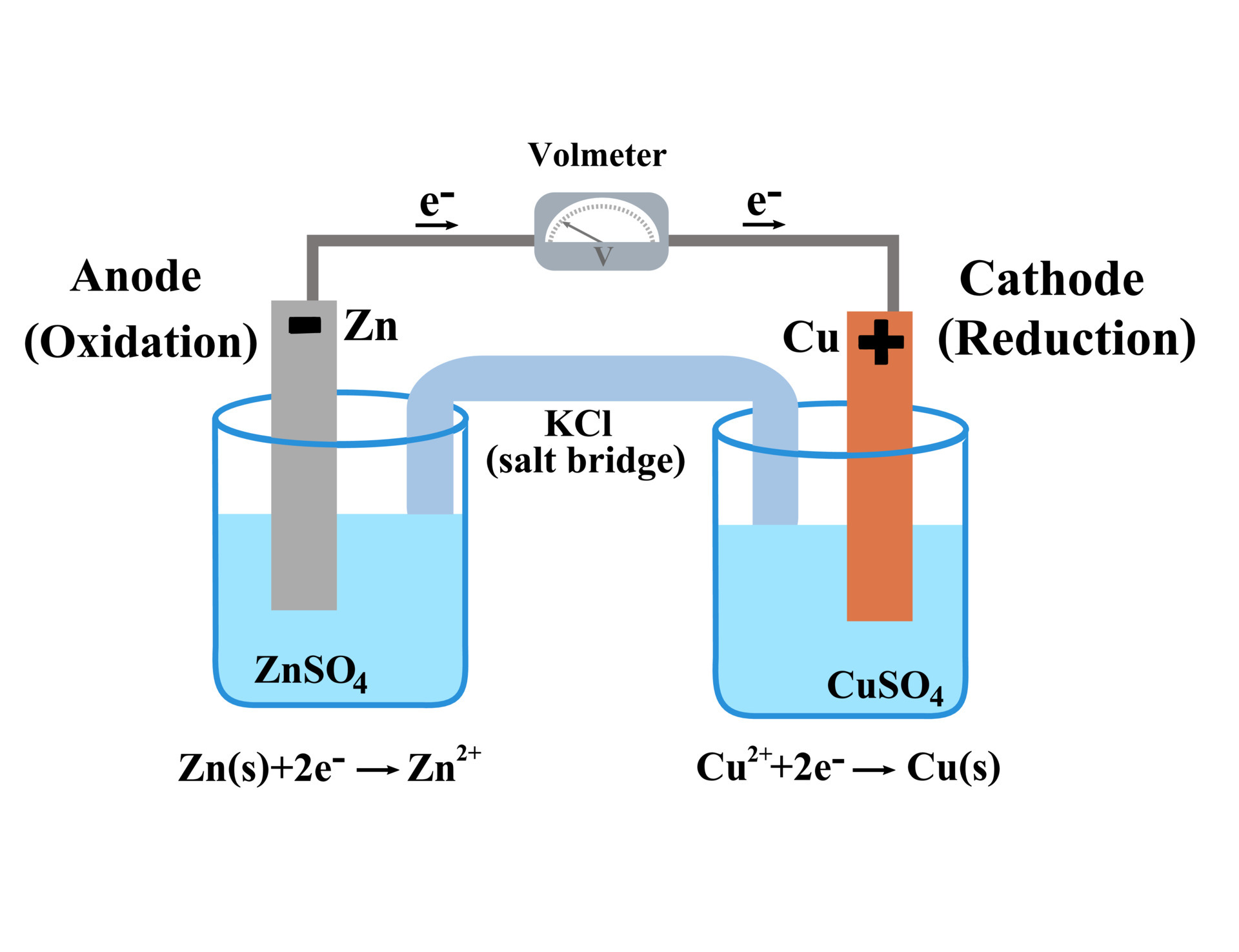
In A Galvanic Cell Reduction Occurs At . A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a polarized electrical device. Consequently an electrical current is made to flow. The cell in which reduction occurs is called the cathode: By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the. A typical galvanic cell, the daniell cell, was used to power telegraphs 100 years ago. The cell in which oxidation occurs is called the anode: A battery is a galvanic cell.
from www.vecteezy.com
A battery is a galvanic cell. The cell in which reduction occurs is called the cathode: The cell in which oxidation occurs is called the anode: A typical galvanic cell, the daniell cell, was used to power telegraphs 100 years ago. A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a polarized electrical device. By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the. Consequently an electrical current is made to flow.
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and
In A Galvanic Cell Reduction Occurs At A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a polarized electrical device. A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a polarized electrical device. The cell in which reduction occurs is called the cathode: The cell in which oxidation occurs is called the anode: By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the. Consequently an electrical current is made to flow. A battery is a galvanic cell. A typical galvanic cell, the daniell cell, was used to power telegraphs 100 years ago.
From mungfali.com
Galvanic And Electrolytic Cells In A Galvanic Cell Reduction Occurs At The cell in which oxidation occurs is called the anode: By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the. The cell in which reduction occurs is called the cathode: Consequently an electrical current is made to flow. A battery is a galvanic cell.. In A Galvanic Cell Reduction Occurs At.
From www.slideserve.com
PPT Galvanic Cells PowerPoint Presentation, free download ID3222668 In A Galvanic Cell Reduction Occurs At By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the. The cell in which oxidation occurs is called the anode: The cell in which reduction occurs is called the cathode: A cathode is defined as an electrode through which positive/negative electric charge flows out. In A Galvanic Cell Reduction Occurs At.
From www.youtube.com
Find the cell potential of a galvanic cell based on the following In A Galvanic Cell Reduction Occurs At The cell in which oxidation occurs is called the anode: A typical galvanic cell, the daniell cell, was used to power telegraphs 100 years ago. By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the. A battery is a galvanic cell. A cathode is. In A Galvanic Cell Reduction Occurs At.
From www.numerade.com
SOLVED A certain halfreaction has standard reduction potential E" 0. In A Galvanic Cell Reduction Occurs At A battery is a galvanic cell. The cell in which oxidation occurs is called the anode: A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a polarized electrical device. A typical galvanic cell, the daniell cell, was used to power telegraphs 100 years ago. By definition, the anode of an electrochemical cell is the. In A Galvanic Cell Reduction Occurs At.
From www.jove.com
Voltaic/Galvanic Cells Principle, Components, Cell Notation (Video) JoVE In A Galvanic Cell Reduction Occurs At A battery is a galvanic cell. A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a polarized electrical device. The cell in which reduction occurs is called the cathode: Consequently an electrical current is made to flow. A typical galvanic cell, the daniell cell, was used to power telegraphs 100 years ago. The cell. In A Galvanic Cell Reduction Occurs At.
From chem.libretexts.org
1 Electrochemical Cells (Experiment) Chemistry LibreTexts In A Galvanic Cell Reduction Occurs At The cell in which reduction occurs is called the cathode: A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a polarized electrical device. The cell in which oxidation occurs is called the anode: A battery is a galvanic cell. By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs. In A Galvanic Cell Reduction Occurs At.
From www.numerade.com
SOLVED ELECTROCHEMISTRY Designing a galvanic cell from two half In A Galvanic Cell Reduction Occurs At The cell in which reduction occurs is called the cathode: A battery is a galvanic cell. A typical galvanic cell, the daniell cell, was used to power telegraphs 100 years ago. By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the. A cathode is. In A Galvanic Cell Reduction Occurs At.
From www.numerade.com
SOLVEDQuestion 32 2 pts In a galvanic cell, reduction occurs at the In A Galvanic Cell Reduction Occurs At Consequently an electrical current is made to flow. A battery is a galvanic cell. The cell in which reduction occurs is called the cathode: The cell in which oxidation occurs is called the anode: By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the.. In A Galvanic Cell Reduction Occurs At.
From www.chemistrylearner.com
Galvanic Cell (Voltaic Cell) Chemistry Learner In A Galvanic Cell Reduction Occurs At A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a polarized electrical device. Consequently an electrical current is made to flow. The cell in which oxidation occurs is called the anode: The cell in which reduction occurs is called the cathode: By definition, the anode of an electrochemical cell is the electrode at which. In A Galvanic Cell Reduction Occurs At.
From www.numerade.com
Galvanic cell based on the above reaction constructed according to the In A Galvanic Cell Reduction Occurs At The cell in which reduction occurs is called the cathode: A battery is a galvanic cell. Consequently an electrical current is made to flow. By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the. The cell in which oxidation occurs is called the anode:. In A Galvanic Cell Reduction Occurs At.
From www.vecteezy.com
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and In A Galvanic Cell Reduction Occurs At The cell in which oxidation occurs is called the anode: A battery is a galvanic cell. A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a polarized electrical device. A typical galvanic cell, the daniell cell, was used to power telegraphs 100 years ago. The cell in which reduction occurs is called the cathode:. In A Galvanic Cell Reduction Occurs At.
From stock.adobe.com
Vettoriale Stock Voltaic galvanic cell or daniell cell.Redox reaction In A Galvanic Cell Reduction Occurs At The cell in which reduction occurs is called the cathode: By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the. A battery is a galvanic cell. A typical galvanic cell, the daniell cell, was used to power telegraphs 100 years ago. A cathode is. In A Galvanic Cell Reduction Occurs At.
From www.tes.com
Galvanic Cells, Anode, Cathode, Oxidation, Reduction, Grade 12 In A Galvanic Cell Reduction Occurs At A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a polarized electrical device. The cell in which oxidation occurs is called the anode: Consequently an electrical current is made to flow. A typical galvanic cell, the daniell cell, was used to power telegraphs 100 years ago. The cell in which reduction occurs is called. In A Galvanic Cell Reduction Occurs At.
From www.numerade.com
SOLVED Suppose the galvanic cell sketched below is powered by the In A Galvanic Cell Reduction Occurs At Consequently an electrical current is made to flow. The cell in which oxidation occurs is called the anode: A battery is a galvanic cell. The cell in which reduction occurs is called the cathode: A typical galvanic cell, the daniell cell, was used to power telegraphs 100 years ago. By definition, the anode of an electrochemical cell is the electrode. In A Galvanic Cell Reduction Occurs At.
From www.chegg.com
Solved I. In a galvanic cell. a. reduction occurs at the In A Galvanic Cell Reduction Occurs At Consequently an electrical current is made to flow. A typical galvanic cell, the daniell cell, was used to power telegraphs 100 years ago. By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the. The cell in which oxidation occurs is called the anode: A. In A Galvanic Cell Reduction Occurs At.
From www.slideserve.com
PPT Voltaic/Galvanic Cells PowerPoint Presentation, free download In A Galvanic Cell Reduction Occurs At A typical galvanic cell, the daniell cell, was used to power telegraphs 100 years ago. A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a polarized electrical device. The cell in which reduction occurs is called the cathode: By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in. In A Galvanic Cell Reduction Occurs At.
From www.slideserve.com
PPT Galvanic (= voltaic) Cells PowerPoint Presentation, free download In A Galvanic Cell Reduction Occurs At Consequently an electrical current is made to flow. The cell in which reduction occurs is called the cathode: A typical galvanic cell, the daniell cell, was used to power telegraphs 100 years ago. The cell in which oxidation occurs is called the anode: A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a polarized. In A Galvanic Cell Reduction Occurs At.
From glossary.periodni.com
Galvanic cell Chemistry Dictionary & Glossary In A Galvanic Cell Reduction Occurs At The cell in which oxidation occurs is called the anode: A battery is a galvanic cell. A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a polarized electrical device. A typical galvanic cell, the daniell cell, was used to power telegraphs 100 years ago. The cell in which reduction occurs is called the cathode:. In A Galvanic Cell Reduction Occurs At.
From joiujlkil.blob.core.windows.net
Standard Cell Potential Galvanic at Rhonda Beecher blog In A Galvanic Cell Reduction Occurs At By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the. A typical galvanic cell, the daniell cell, was used to power telegraphs 100 years ago. The cell in which oxidation occurs is called the anode: A battery is a galvanic cell. A cathode is. In A Galvanic Cell Reduction Occurs At.
From www.numerade.com
SOLVED Using the image of the galvanic cell below, what is the In A Galvanic Cell Reduction Occurs At Consequently an electrical current is made to flow. A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a polarized electrical device. The cell in which reduction occurs is called the cathode: By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the. In A Galvanic Cell Reduction Occurs At.
From www.numerade.com
SOLVED In the galvanic cell, reduction occurs at the and in the In A Galvanic Cell Reduction Occurs At By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the. A battery is a galvanic cell. A typical galvanic cell, the daniell cell, was used to power telegraphs 100 years ago. Consequently an electrical current is made to flow. The cell in which reduction. In A Galvanic Cell Reduction Occurs At.
From allysonkruwnelson.blogspot.com
Where Does Reduction Occur in an Electrochemical Cell AllysonkruwNelson In A Galvanic Cell Reduction Occurs At Consequently an electrical current is made to flow. A typical galvanic cell, the daniell cell, was used to power telegraphs 100 years ago. By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the. A cathode is defined as an electrode through which positive/negative electric. In A Galvanic Cell Reduction Occurs At.
From www.youtube.com
Galvanic Cell Definition, Construction, Working, Example, Diagram In A Galvanic Cell Reduction Occurs At A battery is a galvanic cell. A typical galvanic cell, the daniell cell, was used to power telegraphs 100 years ago. A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a polarized electrical device. Consequently an electrical current is made to flow. The cell in which oxidation occurs is called the anode: By definition,. In A Galvanic Cell Reduction Occurs At.
From www.slideserve.com
PPT Galvanic Cells PowerPoint Presentation, free download ID3222668 In A Galvanic Cell Reduction Occurs At The cell in which reduction occurs is called the cathode: A battery is a galvanic cell. A typical galvanic cell, the daniell cell, was used to power telegraphs 100 years ago. By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the. A cathode is. In A Galvanic Cell Reduction Occurs At.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID1951076 In A Galvanic Cell Reduction Occurs At A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a polarized electrical device. A typical galvanic cell, the daniell cell, was used to power telegraphs 100 years ago. The cell in which reduction occurs is called the cathode: Consequently an electrical current is made to flow. By definition, the anode of an electrochemical cell. In A Galvanic Cell Reduction Occurs At.
From chem.libretexts.org
17.3 Standard Reduction Potentials Chemistry LibreTexts In A Galvanic Cell Reduction Occurs At The cell in which oxidation occurs is called the anode: Consequently an electrical current is made to flow. By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the. The cell in which reduction occurs is called the cathode: A battery is a galvanic cell.. In A Galvanic Cell Reduction Occurs At.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working In A Galvanic Cell Reduction Occurs At The cell in which oxidation occurs is called the anode: The cell in which reduction occurs is called the cathode: A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a polarized electrical device. A typical galvanic cell, the daniell cell, was used to power telegraphs 100 years ago. Consequently an electrical current is made. In A Galvanic Cell Reduction Occurs At.
From www.scienceabc.com
What Are Galvanic Cells? An Oversimplified Explanation » ScienceABC In A Galvanic Cell Reduction Occurs At A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a polarized electrical device. The cell in which oxidation occurs is called the anode: A battery is a galvanic cell. By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is. In A Galvanic Cell Reduction Occurs At.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts In A Galvanic Cell Reduction Occurs At A typical galvanic cell, the daniell cell, was used to power telegraphs 100 years ago. A battery is a galvanic cell. By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the. A cathode is defined as an electrode through which positive/negative electric charge flows. In A Galvanic Cell Reduction Occurs At.
From courses.lumenlearning.com
Galvanic Cells General Chemistry In A Galvanic Cell Reduction Occurs At Consequently an electrical current is made to flow. The cell in which oxidation occurs is called the anode: A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a polarized electrical device. A typical galvanic cell, the daniell cell, was used to power telegraphs 100 years ago. The cell in which reduction occurs is called. In A Galvanic Cell Reduction Occurs At.
From www.geeksforgeeks.org
Galvanic Cell Definition, Construction, Working Principle In A Galvanic Cell Reduction Occurs At A battery is a galvanic cell. A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a polarized electrical device. A typical galvanic cell, the daniell cell, was used to power telegraphs 100 years ago. The cell in which reduction occurs is called the cathode: The cell in which oxidation occurs is called the anode:. In A Galvanic Cell Reduction Occurs At.
From www.numerade.com
SOLVED What is galvanic cell? 2. In galvanic cell, at which electrode In A Galvanic Cell Reduction Occurs At The cell in which oxidation occurs is called the anode: Consequently an electrical current is made to flow. A typical galvanic cell, the daniell cell, was used to power telegraphs 100 years ago. A battery is a galvanic cell. The cell in which reduction occurs is called the cathode: A cathode is defined as an electrode through which positive/negative electric. In A Galvanic Cell Reduction Occurs At.
From www.chegg.com
Solved A galvanic cell is powered by the following redox In A Galvanic Cell Reduction Occurs At A cathode is defined as an electrode through which positive/negative electric charge flows out of/into a polarized electrical device. A typical galvanic cell, the daniell cell, was used to power telegraphs 100 years ago. The cell in which reduction occurs is called the cathode: A battery is a galvanic cell. By definition, the anode of an electrochemical cell is the. In A Galvanic Cell Reduction Occurs At.
From general.chemistrysteps.com
Galvanic Cells Chemistry Steps In A Galvanic Cell Reduction Occurs At Consequently an electrical current is made to flow. A battery is a galvanic cell. The cell in which reduction occurs is called the cathode: By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the. A typical galvanic cell, the daniell cell, was used to. In A Galvanic Cell Reduction Occurs At.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working In A Galvanic Cell Reduction Occurs At A typical galvanic cell, the daniell cell, was used to power telegraphs 100 years ago. By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the. A battery is a galvanic cell. A cathode is defined as an electrode through which positive/negative electric charge flows. In A Galvanic Cell Reduction Occurs At.