Standard Enthalpy Of Formation P4O10 . The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. C(s) + o 2(g) co 2(g) δh ∘ f. Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as. The standard enthalpy of formation of co 2 (g) is −393.5 kj/mol. This is the enthalpy change for the exothermic reaction: Consider the following standard heats of formation: 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,.
from www.chegg.com
The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. This is the enthalpy change for the exothermic reaction: C(s) + o 2(g) co 2(g) δh ∘ f. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation of co 2 (g) is −393.5 kj/mol. Consider the following standard heats of formation: Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as.
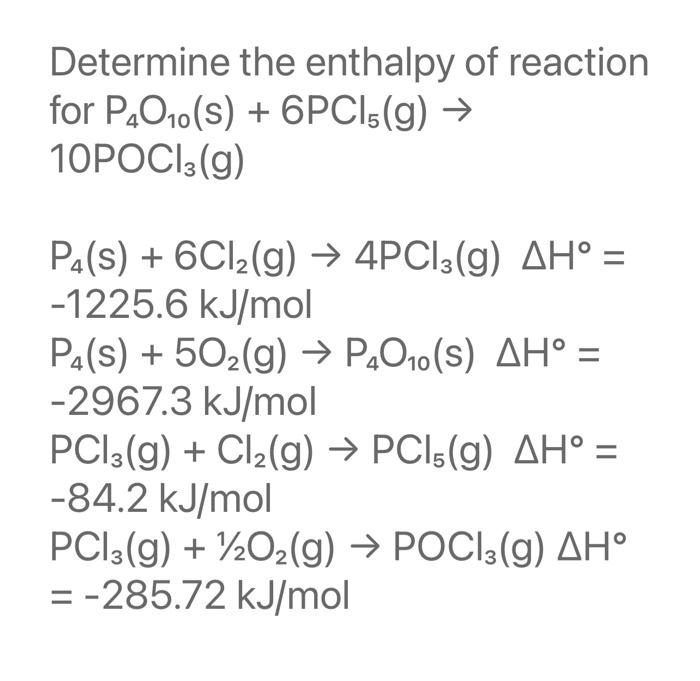
Solved Determine the enthalpy of reaction for P4O10(
Standard Enthalpy Of Formation P4O10 This is the enthalpy change for the exothermic reaction: Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation of co 2 (g) is −393.5 kj/mol. This is the enthalpy change for the exothermic reaction: C(s) + o 2(g) co 2(g) δh ∘ f. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Consider the following standard heats of formation: The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard.
From pdfprof.com
enthalpies standard de formation et entropie standard Standard Enthalpy Of Formation P4O10 This is the enthalpy change for the exothermic reaction: Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as. C(s) + o 2(g) co 2(g) δh ∘ f. Consider the following standard heats of formation: The standard enthalpy of formation of co 2 (g) is −393.5 kj/mol.. Standard Enthalpy Of Formation P4O10.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Enthalpy Of Formation P4O10 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. C(s) + o 2(g) co 2(g) δh ∘ f. Consider the following standard heats of formation: This is the enthalpy change for the exothermic reaction: 136 rows standard enthalpy change of formation (data table) these tables include. Standard Enthalpy Of Formation P4O10.
From bceweb.org
Enthalpy Of Formation Chart A Visual Reference of Charts Chart Master Standard Enthalpy Of Formation P4O10 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Enthalpy of formation (δhf) is the enthalpy change for the formation of 1. Standard Enthalpy Of Formation P4O10.
From narodnatribuna.info
Calculating Reaction Enthalpy From Enthalpies Of Formation Standard Enthalpy Of Formation P4O10 Consider the following standard heats of formation: Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a. Standard Enthalpy Of Formation P4O10.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all Standard Enthalpy Of Formation P4O10 This is the enthalpy change for the exothermic reaction: C(s) + o 2(g) co 2(g) δh ∘ f. The standard enthalpy of formation of co 2 (g) is −393.5 kj/mol. Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as. Consider the following standard heats of formation:. Standard Enthalpy Of Formation P4O10.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Standard Enthalpy Of Formation P4O10 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Consider the following standard heats of formation: The standard enthalpy of formation of co 2 (g) is −393.5 kj/mol. This is the enthalpy change for the exothermic reaction: Enthalpy of formation (δhf) is the enthalpy change for. Standard Enthalpy Of Formation P4O10.
From www.youtube.com
How to Calculate the Standard Enthalpy of a Reaction from the Standard Standard Enthalpy Of Formation P4O10 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Consider the following standard heats of formation: The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. This is the enthalpy change for the. Standard Enthalpy Of Formation P4O10.
From www.chegg.com
Solved Determine the enthalpy of reaction for P4O10( Standard Enthalpy Of Formation P4O10 C(s) + o 2(g) co 2(g) δh ∘ f. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Consider the following standard. Standard Enthalpy Of Formation P4O10.
From www.numerade.com
SOLVED The standard enthalpy change of formation values of two oxides Standard Enthalpy Of Formation P4O10 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. C(s) + o 2(g) co 2(g) δh ∘ f. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Consider the following standard heats of. Standard Enthalpy Of Formation P4O10.
From www.chemistryspace.com
Standard Enthalpy of Formation Standard Enthalpy Of Formation P4O10 Consider the following standard heats of formation: The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. The standard enthalpy of formation of co. Standard Enthalpy Of Formation P4O10.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Enthalpy Of Formation P4O10 Consider the following standard heats of formation: 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. The standard enthalpy of formation of co 2 (g) is −393.5 kj/mol. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a. Standard Enthalpy Of Formation P4O10.
From schoolworkhelper.net
Standard Enthalpies of Formation Online Homework Help SchoolWorkHelper Standard Enthalpy Of Formation P4O10 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Consider the following standard heats of formation: C(s) + o 2(g) co 2(g) δh ∘. Standard Enthalpy Of Formation P4O10.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Standard Enthalpy Of Formation P4O10 C(s) + o 2(g) co 2(g) δh ∘ f. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. The standard enthalpy of formation. Standard Enthalpy Of Formation P4O10.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Standard Enthalpy Of Formation P4O10 Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as. The standard enthalpy of formation of co 2 (g) is −393.5 kj/mol. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Consider the. Standard Enthalpy Of Formation P4O10.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation P4O10 This is the enthalpy change for the exothermic reaction: 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. C(s) + o 2(g) co 2(g) δh ∘ f. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. Standard Enthalpy Of Formation P4O10.
From studylib.net
I. Standard Enthalpies of Formation Standard Enthalpy Of Formation P4O10 Consider the following standard heats of formation: The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation of co 2 (g) is −393.5 kj/mol. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered. Standard Enthalpy Of Formation P4O10.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Standard Enthalpy Of Formation P4O10 The standard enthalpy of formation of co 2 (g) is −393.5 kj/mol. Consider the following standard heats of formation: This is the enthalpy change for the exothermic reaction: The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. C(s) + o 2(g) co 2(g) δh ∘. Standard Enthalpy Of Formation P4O10.
From mavink.com
Standard Enthalpy Chart Standard Enthalpy Of Formation P4O10 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. C(s) + o 2(g) co 2(g) δh ∘ f. Consider the following standard heats of formation: Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such. Standard Enthalpy Of Formation P4O10.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube Standard Enthalpy Of Formation P4O10 The standard enthalpy of formation of co 2 (g) is −393.5 kj/mol. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. This. Standard Enthalpy Of Formation P4O10.
From www.nagwa.com
Question Video Calculating the Standard Enthalpy of Formation for Standard Enthalpy Of Formation P4O10 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. This is the enthalpy change for the exothermic reaction: Consider the following standard heats of. Standard Enthalpy Of Formation P4O10.
From www.slideshare.net
Standard enthalpy of formation Standard Enthalpy Of Formation P4O10 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. This is the enthalpy change for the exothermic reaction: Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as. 193 rows in chemistry and thermodynamics,. Standard Enthalpy Of Formation P4O10.
From www.youtube.com
How to Balance P + O2 = P4O10 (Phosphorus + Oxygen gas) YouTube Standard Enthalpy Of Formation P4O10 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Consider the following standard heats of formation: The standard enthalpy of formation of co 2 (g) is −393.5 kj/mol. C(s) + o 2(g) co 2(g) δh ∘ f. The standard enthalpy of formation is a measure of the. Standard Enthalpy Of Formation P4O10.
From www.numerade.com
SOLVED A scientist measures the standard enthalpy change for the Standard Enthalpy Of Formation P4O10 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. This is the enthalpy change for the exothermic reaction: The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of. Standard Enthalpy Of Formation P4O10.
From www.numerade.com
SOLVED Calculate the standard enthalpy change for the following Standard Enthalpy Of Formation P4O10 Consider the following standard heats of formation: C(s) + o 2(g) co 2(g) δh ∘ f. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of. Standard Enthalpy Of Formation P4O10.
From www.chegg.com
Solved Determine the enthalpy of reaction for P4O10( Standard Enthalpy Of Formation P4O10 The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. C(s) + o 2(g) co 2(g) δh ∘ f. Consider the following standard heats. Standard Enthalpy Of Formation P4O10.
From www.numerade.com
SOLVED Given the following reaction P4O10(s) + 6 H2O(l) = 4 H3PO4 (aq Standard Enthalpy Of Formation P4O10 C(s) + o 2(g) co 2(g) δh ∘ f. Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as. The standard enthalpy of formation of co 2 (g) is −393.5 kj/mol. Consider the following standard heats of formation: The standard enthalpy of formation is a measure of. Standard Enthalpy Of Formation P4O10.
From www.studocu.com
Standard Enthalpy of Formation Table Standard Enthalpy of Formation Standard Enthalpy Of Formation P4O10 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. This is the enthalpy change for the exothermic reaction: 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation of. Standard Enthalpy Of Formation P4O10.
From mungfali.com
Enthalpies Of Formation Chart Standard Enthalpy Of Formation P4O10 The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. This is the enthalpy change for the exothermic reaction: The standard enthalpy of formation of co 2 (g) is −393.5 kj/mol. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation. Standard Enthalpy Of Formation P4O10.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation P4O10 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. C(s) + o 2(g) co 2(g) δh ∘ f. Consider the following standard heats. Standard Enthalpy Of Formation P4O10.
From mungfali.com
Enthalpies Of Formation Table Standard Enthalpy Of Formation P4O10 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. C(s) + o 2(g) co 2(g) δh ∘ f. The standard enthalpy of formation of. Standard Enthalpy Of Formation P4O10.
From www.slideserve.com
PPT Chemical Thermodynamics PowerPoint Presentation, free download Standard Enthalpy Of Formation P4O10 Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as. This is the enthalpy change for the exothermic reaction: The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Consider the following standard. Standard Enthalpy Of Formation P4O10.
From www.chegg.com
Solved Use the standard enthalpies of formation in the table Standard Enthalpy Of Formation P4O10 Consider the following standard heats of formation: 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. C(s) + o 2(g) co 2(g) δh ∘ f. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. Standard Enthalpy Of Formation P4O10.
From stahonorschemistry.weebly.com
III Calculating Enthalpies STA Form IV Honors Chemistry Standard Enthalpy Of Formation P4O10 The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. The standard enthalpy of formation of co 2 (g) is −393.5 kj/mol. C(s) +. Standard Enthalpy Of Formation P4O10.
From www.toppr.com
Stepwise hydrolysis of P4O10 takes place via formation of Standard Enthalpy Of Formation P4O10 C(s) + o 2(g) co 2(g) δh ∘ f. The standard enthalpy of formation of co 2 (g) is −393.5 kj/mol. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Consider the following standard heats of formation: This is the enthalpy change for the exothermic. Standard Enthalpy Of Formation P4O10.
From www.numerade.com
SOLVED Question 24 What is the enthalpy change for the reaction, P4O6 Standard Enthalpy Of Formation P4O10 Consider the following standard heats of formation: The standard enthalpy of formation of co 2 (g) is −393.5 kj/mol. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. This is the enthalpy change for the exothermic reaction: 193 rows in chemistry and thermodynamics, the standard enthalpy of. Standard Enthalpy Of Formation P4O10.