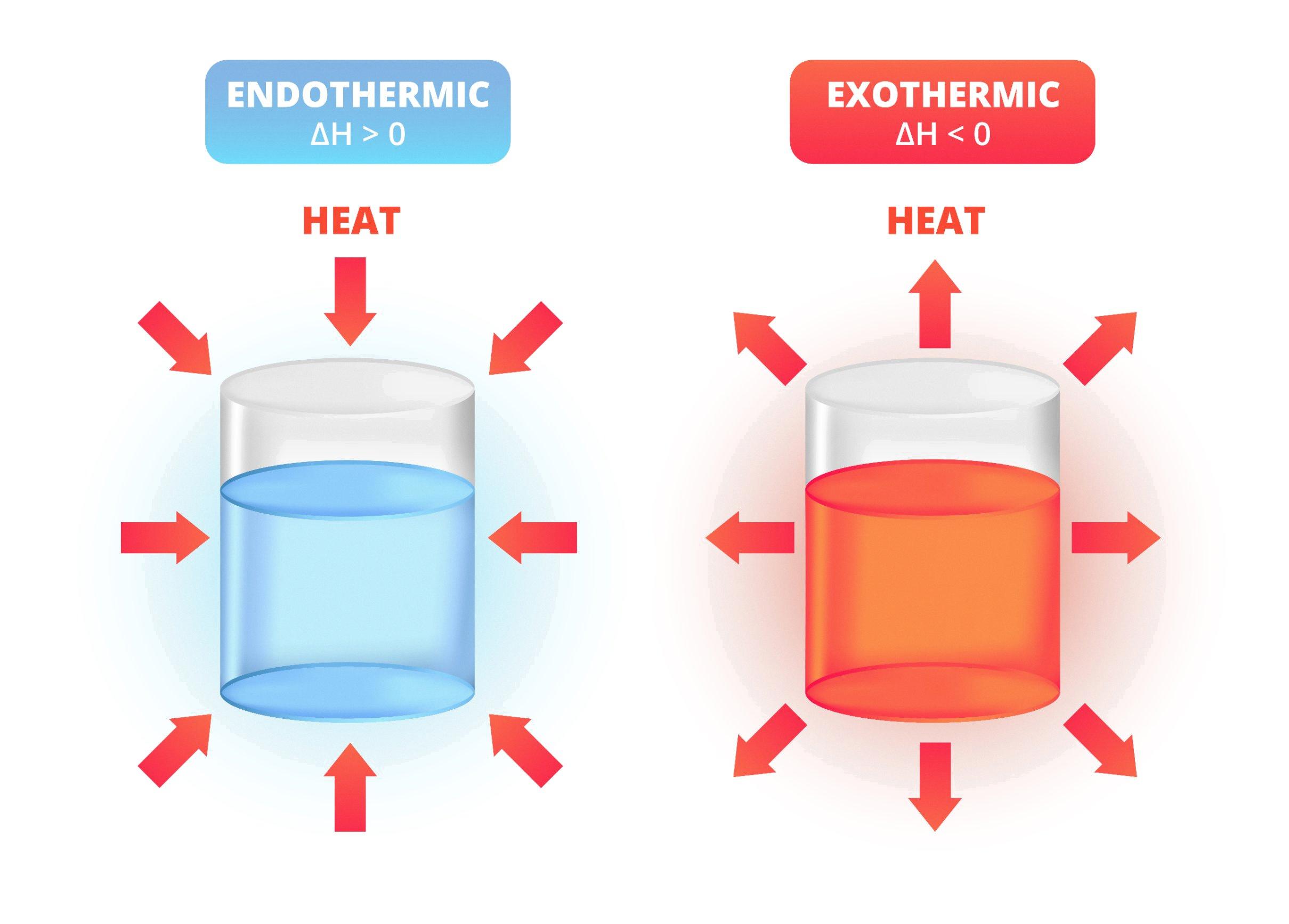
Melting Candle Wax Endothermic Exothermic . The wax (c 34 h 70) combusts in the presence of oxygen (o 2) to yield carbon dioxide (co. As wax is consumed, capillary action draws more liquid wax along the wick. the graph below charts the energy change when a candle burns. This process makes the immediate air around the flame very dry as the moisture from the candle is evaporated. the melting of candle wax is an endothermic change because it absorbs energy from its surroundings to break the. The heat of the flame vaporizes the wax molecules and they react with the oxygen in the air. to determine if a candle burning is exothermic or endothermic we need. when the air surrounding the candle is heated, it sets up an evaporation process of the water particles released during the melting of the candle wax. when you light a candle, wax near the wick melts into a liquid.
from h-o-m-e.org
when you light a candle, wax near the wick melts into a liquid. As wax is consumed, capillary action draws more liquid wax along the wick. to determine if a candle burning is exothermic or endothermic we need. the melting of candle wax is an endothermic change because it absorbs energy from its surroundings to break the. The heat of the flame vaporizes the wax molecules and they react with the oxygen in the air. when the air surrounding the candle is heated, it sets up an evaporation process of the water particles released during the melting of the candle wax. The wax (c 34 h 70) combusts in the presence of oxygen (o 2) to yield carbon dioxide (co. This process makes the immediate air around the flame very dry as the moisture from the candle is evaporated. the graph below charts the energy change when a candle burns.
Endothermic Reactions The Science Behind Temperature Change
Melting Candle Wax Endothermic Exothermic when you light a candle, wax near the wick melts into a liquid. As wax is consumed, capillary action draws more liquid wax along the wick. when the air surrounding the candle is heated, it sets up an evaporation process of the water particles released during the melting of the candle wax. when you light a candle, wax near the wick melts into a liquid. The heat of the flame vaporizes the wax molecules and they react with the oxygen in the air. the melting of candle wax is an endothermic change because it absorbs energy from its surroundings to break the. The wax (c 34 h 70) combusts in the presence of oxygen (o 2) to yield carbon dioxide (co. to determine if a candle burning is exothermic or endothermic we need. the graph below charts the energy change when a candle burns. This process makes the immediate air around the flame very dry as the moisture from the candle is evaporated.
From kunduz.com
Exothermic Reaction Definition, Properties, Examples, Exothermic and Melting Candle Wax Endothermic Exothermic This process makes the immediate air around the flame very dry as the moisture from the candle is evaporated. The wax (c 34 h 70) combusts in the presence of oxygen (o 2) to yield carbon dioxide (co. The heat of the flame vaporizes the wax molecules and they react with the oxygen in the air. the graph below. Melting Candle Wax Endothermic Exothermic.
From slideplayer.com
Exothermic and Endothermic Reactions ppt download Melting Candle Wax Endothermic Exothermic This process makes the immediate air around the flame very dry as the moisture from the candle is evaporated. to determine if a candle burning is exothermic or endothermic we need. when the air surrounding the candle is heated, it sets up an evaporation process of the water particles released during the melting of the candle wax. . Melting Candle Wax Endothermic Exothermic.
From www.inf-inet.com
How To Melt Candle Wax Melting Candle Wax Endothermic Exothermic to determine if a candle burning is exothermic or endothermic we need. The wax (c 34 h 70) combusts in the presence of oxygen (o 2) to yield carbon dioxide (co. The heat of the flame vaporizes the wax molecules and they react with the oxygen in the air. when you light a candle, wax near the wick. Melting Candle Wax Endothermic Exothermic.
From slideplayer.com
THERMODYNAMICS Intro & Calorimetry. ppt download Melting Candle Wax Endothermic Exothermic The wax (c 34 h 70) combusts in the presence of oxygen (o 2) to yield carbon dioxide (co. The heat of the flame vaporizes the wax molecules and they react with the oxygen in the air. the graph below charts the energy change when a candle burns. to determine if a candle burning is exothermic or endothermic. Melting Candle Wax Endothermic Exothermic.
From www.candleers.com
At What Temperature Does Candle Wax Melt (Wax Melting Point) Melting Candle Wax Endothermic Exothermic the graph below charts the energy change when a candle burns. As wax is consumed, capillary action draws more liquid wax along the wick. This process makes the immediate air around the flame very dry as the moisture from the candle is evaporated. the melting of candle wax is an endothermic change because it absorbs energy from its. Melting Candle Wax Endothermic Exothermic.
From mungfali.com
Exothermic Vs Endothermic Diagram Melting Candle Wax Endothermic Exothermic As wax is consumed, capillary action draws more liquid wax along the wick. when the air surrounding the candle is heated, it sets up an evaporation process of the water particles released during the melting of the candle wax. The heat of the flame vaporizes the wax molecules and they react with the oxygen in the air. This process. Melting Candle Wax Endothermic Exothermic.
From fixlibrarynadred27.z14.web.core.windows.net
Exothermic And Endothermic Diagrams Melting Candle Wax Endothermic Exothermic the graph below charts the energy change when a candle burns. The heat of the flame vaporizes the wax molecules and they react with the oxygen in the air. This process makes the immediate air around the flame very dry as the moisture from the candle is evaporated. to determine if a candle burning is exothermic or endothermic. Melting Candle Wax Endothermic Exothermic.
From www.slideserve.com
PPT Phases of Matter PowerPoint Presentation ID3368004 Melting Candle Wax Endothermic Exothermic As wax is consumed, capillary action draws more liquid wax along the wick. when the air surrounding the candle is heated, it sets up an evaporation process of the water particles released during the melting of the candle wax. This process makes the immediate air around the flame very dry as the moisture from the candle is evaporated. . Melting Candle Wax Endothermic Exothermic.
From muadacsan3mien.com
What Are Exothermic And Endothermic Changes? Examples Unveiled! Melting Candle Wax Endothermic Exothermic to determine if a candle burning is exothermic or endothermic we need. This process makes the immediate air around the flame very dry as the moisture from the candle is evaporated. As wax is consumed, capillary action draws more liquid wax along the wick. when the air surrounding the candle is heated, it sets up an evaporation process. Melting Candle Wax Endothermic Exothermic.
From h-o-m-e.org
Endothermic Reactions The Science Behind Temperature Change Melting Candle Wax Endothermic Exothermic when you light a candle, wax near the wick melts into a liquid. the graph below charts the energy change when a candle burns. This process makes the immediate air around the flame very dry as the moisture from the candle is evaporated. As wax is consumed, capillary action draws more liquid wax along the wick. when. Melting Candle Wax Endothermic Exothermic.
From vhmsscience8.weebly.com
Candle Lab VISTA HEIGHTS 8TH GRADE SCIENCE Melting Candle Wax Endothermic Exothermic the graph below charts the energy change when a candle burns. This process makes the immediate air around the flame very dry as the moisture from the candle is evaporated. to determine if a candle burning is exothermic or endothermic we need. the melting of candle wax is an endothermic change because it absorbs energy from its. Melting Candle Wax Endothermic Exothermic.
From www.slideserve.com
PPT Endothermic or Exothermic? Melting ice Endothermic PowerPoint Melting Candle Wax Endothermic Exothermic the melting of candle wax is an endothermic change because it absorbs energy from its surroundings to break the. when you light a candle, wax near the wick melts into a liquid. The heat of the flame vaporizes the wax molecules and they react with the oxygen in the air. As wax is consumed, capillary action draws more. Melting Candle Wax Endothermic Exothermic.
From byjus.com
Difference Between Endothermic and Exothermic Reactions Chemistry Melting Candle Wax Endothermic Exothermic when the air surrounding the candle is heated, it sets up an evaporation process of the water particles released during the melting of the candle wax. to determine if a candle burning is exothermic or endothermic we need. the melting of candle wax is an endothermic change because it absorbs energy from its surroundings to break the.. Melting Candle Wax Endothermic Exothermic.
From scentsationals.com
Wax Melt Warmers vs. Fragrance Candles ScentSationals Melting Candle Wax Endothermic Exothermic the melting of candle wax is an endothermic change because it absorbs energy from its surroundings to break the. to determine if a candle burning is exothermic or endothermic we need. The wax (c 34 h 70) combusts in the presence of oxygen (o 2) to yield carbon dioxide (co. the graph below charts the energy change. Melting Candle Wax Endothermic Exothermic.
From slideplayer.com
Thermal Energy & Heat Chapter 2 Section 3 5. Reactions ppt download Melting Candle Wax Endothermic Exothermic to determine if a candle burning is exothermic or endothermic we need. the graph below charts the energy change when a candle burns. As wax is consumed, capillary action draws more liquid wax along the wick. when you light a candle, wax near the wick melts into a liquid. The heat of the flame vaporizes the wax. Melting Candle Wax Endothermic Exothermic.
From exojeofwi.blob.core.windows.net
What Temperature To Melt Candle Wax at Jacob Wilkerson blog Melting Candle Wax Endothermic Exothermic the melting of candle wax is an endothermic change because it absorbs energy from its surroundings to break the. The wax (c 34 h 70) combusts in the presence of oxygen (o 2) to yield carbon dioxide (co. when you light a candle, wax near the wick melts into a liquid. The heat of the flame vaporizes the. Melting Candle Wax Endothermic Exothermic.
From studyschoolnucleator.z21.web.core.windows.net
Identify Endothermic And Exothermic Reactions Melting Candle Wax Endothermic Exothermic the graph below charts the energy change when a candle burns. when you light a candle, wax near the wick melts into a liquid. when the air surrounding the candle is heated, it sets up an evaporation process of the water particles released during the melting of the candle wax. As wax is consumed, capillary action draws. Melting Candle Wax Endothermic Exothermic.
From neocandle.com
What Temperature Does Candle Wax Melt At? Find Out Here! Melting Candle Wax Endothermic Exothermic This process makes the immediate air around the flame very dry as the moisture from the candle is evaporated. the graph below charts the energy change when a candle burns. when you light a candle, wax near the wick melts into a liquid. The heat of the flame vaporizes the wax molecules and they react with the oxygen. Melting Candle Wax Endothermic Exothermic.
From manuallistgelsemine.z13.web.core.windows.net
Exothermic And Endothermic Diagrams Melting Candle Wax Endothermic Exothermic when the air surrounding the candle is heated, it sets up an evaporation process of the water particles released during the melting of the candle wax. The heat of the flame vaporizes the wax molecules and they react with the oxygen in the air. As wax is consumed, capillary action draws more liquid wax along the wick. the. Melting Candle Wax Endothermic Exothermic.
From www.worksheetsplanet.com
What is an Exothermic Reaction Definition and Example Melting Candle Wax Endothermic Exothermic The heat of the flame vaporizes the wax molecules and they react with the oxygen in the air. when you light a candle, wax near the wick melts into a liquid. As wax is consumed, capillary action draws more liquid wax along the wick. to determine if a candle burning is exothermic or endothermic we need. when. Melting Candle Wax Endothermic Exothermic.
From lessonschoolallodial.z13.web.core.windows.net
Exothermic And Endothermic Reaction Example Melting Candle Wax Endothermic Exothermic when you light a candle, wax near the wick melts into a liquid. when the air surrounding the candle is heated, it sets up an evaporation process of the water particles released during the melting of the candle wax. the melting of candle wax is an endothermic change because it absorbs energy from its surroundings to break. Melting Candle Wax Endothermic Exothermic.
From devan-bloghuang.blogspot.com
Chemical Properties of Candle Wax Melting Candle Wax Endothermic Exothermic This process makes the immediate air around the flame very dry as the moisture from the candle is evaporated. the graph below charts the energy change when a candle burns. when the air surrounding the candle is heated, it sets up an evaporation process of the water particles released during the melting of the candle wax. when. Melting Candle Wax Endothermic Exothermic.
From slideplayer.com
Chapter 17 Thermochemistry ppt download Melting Candle Wax Endothermic Exothermic the graph below charts the energy change when a candle burns. As wax is consumed, capillary action draws more liquid wax along the wick. when you light a candle, wax near the wick melts into a liquid. when the air surrounding the candle is heated, it sets up an evaporation process of the water particles released during. Melting Candle Wax Endothermic Exothermic.
From www.tes.com
Exothermic and Endothermic Reactions Teaching Resources Melting Candle Wax Endothermic Exothermic The heat of the flame vaporizes the wax molecules and they react with the oxygen in the air. to determine if a candle burning is exothermic or endothermic we need. As wax is consumed, capillary action draws more liquid wax along the wick. the melting of candle wax is an endothermic change because it absorbs energy from its. Melting Candle Wax Endothermic Exothermic.
From www.savemyexams.com
Exothermic & Endothermic Edexcel IGCSE Chemistry Revision Notes 2019 Melting Candle Wax Endothermic Exothermic the melting of candle wax is an endothermic change because it absorbs energy from its surroundings to break the. to determine if a candle burning is exothermic or endothermic we need. The heat of the flame vaporizes the wax molecules and they react with the oxygen in the air. when you light a candle, wax near the. Melting Candle Wax Endothermic Exothermic.
From www.difference101.com
Endothermic vs. Exothermic 5 Key Differences, Pros & Cons, Examples Melting Candle Wax Endothermic Exothermic the graph below charts the energy change when a candle burns. the melting of candle wax is an endothermic change because it absorbs energy from its surroundings to break the. As wax is consumed, capillary action draws more liquid wax along the wick. when the air surrounding the candle is heated, it sets up an evaporation process. Melting Candle Wax Endothermic Exothermic.
From www.pinterest.ph
Endothermic and Exothermic Reactions infographic diagram showing Melting Candle Wax Endothermic Exothermic the graph below charts the energy change when a candle burns. The wax (c 34 h 70) combusts in the presence of oxygen (o 2) to yield carbon dioxide (co. The heat of the flame vaporizes the wax molecules and they react with the oxygen in the air. to determine if a candle burning is exothermic or endothermic. Melting Candle Wax Endothermic Exothermic.
From printablelibswathed.z19.web.core.windows.net
Exothermic And Endothermic Reaction Example Melting Candle Wax Endothermic Exothermic The wax (c 34 h 70) combusts in the presence of oxygen (o 2) to yield carbon dioxide (co. the melting of candle wax is an endothermic change because it absorbs energy from its surroundings to break the. This process makes the immediate air around the flame very dry as the moisture from the candle is evaporated. As wax. Melting Candle Wax Endothermic Exothermic.
From www.worksheetsplanet.com
What is an Endothermic Reaction Definition & Example Melting Candle Wax Endothermic Exothermic the melting of candle wax is an endothermic change because it absorbs energy from its surroundings to break the. when you light a candle, wax near the wick melts into a liquid. As wax is consumed, capillary action draws more liquid wax along the wick. to determine if a candle burning is exothermic or endothermic we need.. Melting Candle Wax Endothermic Exothermic.
From mungfali.com
Endothermic Vs Exothermic Reactions Examples Melting Candle Wax Endothermic Exothermic when the air surrounding the candle is heated, it sets up an evaporation process of the water particles released during the melting of the candle wax. when you light a candle, wax near the wick melts into a liquid. This process makes the immediate air around the flame very dry as the moisture from the candle is evaporated.. Melting Candle Wax Endothermic Exothermic.
From studybrewmaster.z21.web.core.windows.net
Why Is Condensation Exothermic Melting Candle Wax Endothermic Exothermic the graph below charts the energy change when a candle burns. the melting of candle wax is an endothermic change because it absorbs energy from its surroundings to break the. The heat of the flame vaporizes the wax molecules and they react with the oxygen in the air. when you light a candle, wax near the wick. Melting Candle Wax Endothermic Exothermic.
From whatisdiffer.com
Difference Between Endothermic And Exothermic Reactions? Melting Candle Wax Endothermic Exothermic the melting of candle wax is an endothermic change because it absorbs energy from its surroundings to break the. the graph below charts the energy change when a candle burns. when the air surrounding the candle is heated, it sets up an evaporation process of the water particles released during the melting of the candle wax. As. Melting Candle Wax Endothermic Exothermic.
From mmerevise.co.uk
Endothermic and Exothermic Reactions Revision MME Melting Candle Wax Endothermic Exothermic the melting of candle wax is an endothermic change because it absorbs energy from its surroundings to break the. to determine if a candle burning is exothermic or endothermic we need. The wax (c 34 h 70) combusts in the presence of oxygen (o 2) to yield carbon dioxide (co. the graph below charts the energy change. Melting Candle Wax Endothermic Exothermic.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE Melting Candle Wax Endothermic Exothermic The heat of the flame vaporizes the wax molecules and they react with the oxygen in the air. The wax (c 34 h 70) combusts in the presence of oxygen (o 2) to yield carbon dioxide (co. the melting of candle wax is an endothermic change because it absorbs energy from its surroundings to break the. to determine. Melting Candle Wax Endothermic Exothermic.
From whatsinsight.org
Endothermic vs. Exothermic with Examples What's Insight Melting Candle Wax Endothermic Exothermic the melting of candle wax is an endothermic change because it absorbs energy from its surroundings to break the. to determine if a candle burning is exothermic or endothermic we need. The wax (c 34 h 70) combusts in the presence of oxygen (o 2) to yield carbon dioxide (co. the graph below charts the energy change. Melting Candle Wax Endothermic Exothermic.