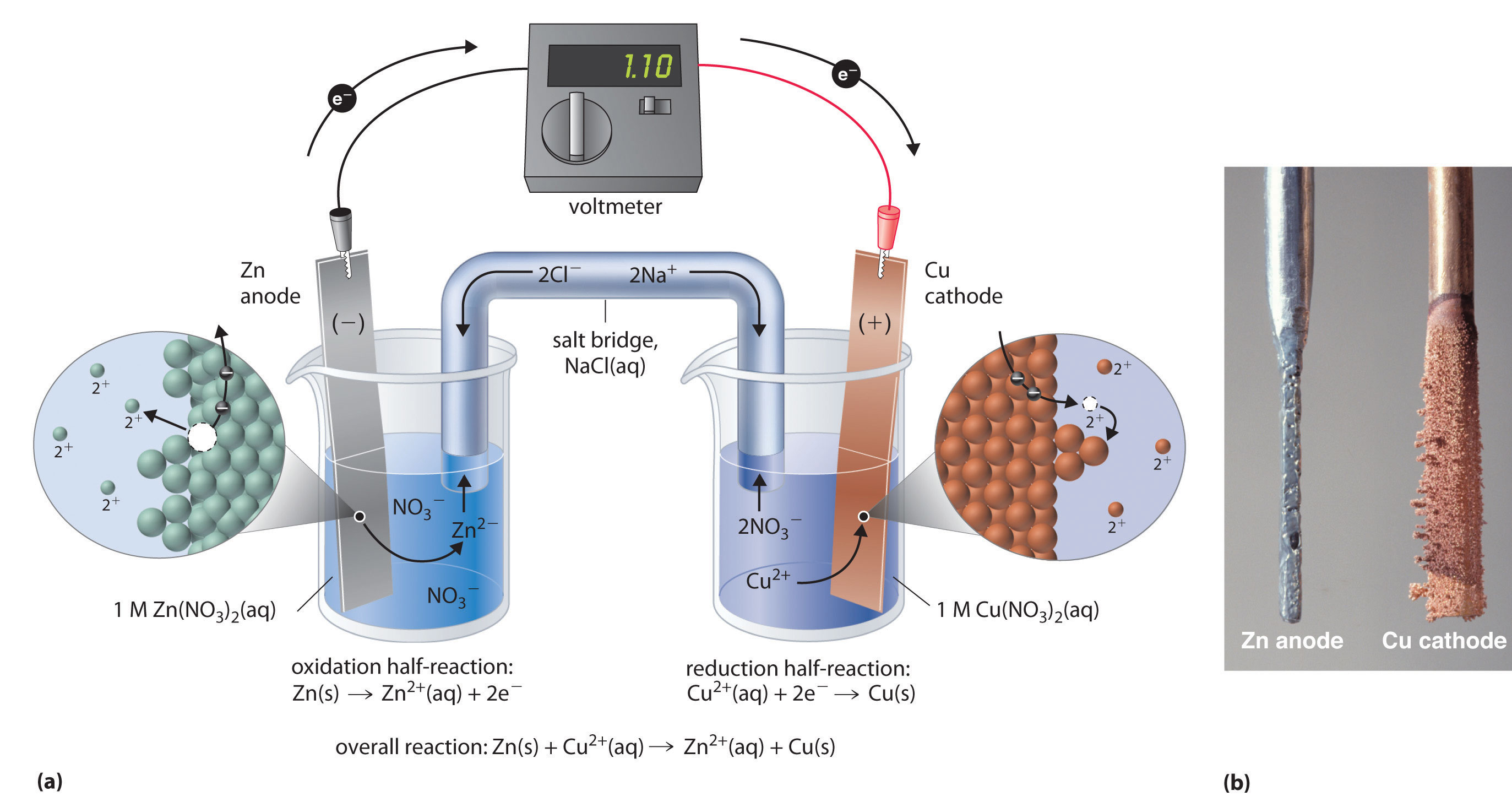
The Electrode In A Galvanic Cell At Which Oxidation Occurs . by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag foil). The name refers to the flow of anions in the. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode.
from 2012books.lardbucket.org
by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag foil). The name refers to the flow of anions in the. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode.
Electrochemistry
The Electrode In A Galvanic Cell At Which Oxidation Occurs by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the. The name refers to the flow of anions in the. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag foil).
From dxodcnqen.blob.core.windows.net
Standard Hydrogen Electrode In A Galvanic Cell at Katharine Murphy blog The Electrode In A Galvanic Cell At Which Oxidation Occurs by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the. The Electrode In A Galvanic Cell At Which Oxidation Occurs.
From www.alamy.com
Illustration of a voltaic cell, or galvanic cell, an electrochemical The Electrode In A Galvanic Cell At Which Oxidation Occurs by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag foil). by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is. The Electrode In A Galvanic Cell At Which Oxidation Occurs.
From general.chemistrysteps.com
Galvanic Cells Chemistry Steps The Electrode In A Galvanic Cell At Which Oxidation Occurs The name refers to the flow of anions in the. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil). The Electrode In A Galvanic Cell At Which Oxidation Occurs.
From cider.uoregon.edu
Voltaic Galvanic Cell (Electrochemical Cell) Simulation AACT CIDER The Electrode In A Galvanic Cell At Which Oxidation Occurs by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the. The Electrode In A Galvanic Cell At Which Oxidation Occurs.
From chem.libretexts.org
11.1 Galvanic Cells Chemistry LibreTexts The Electrode In A Galvanic Cell At Which Oxidation Occurs by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode. by definition, the. The Electrode In A Galvanic Cell At Which Oxidation Occurs.
From schoolbag.info
Electrochemical Cells Electrochemistry Training MCAT General The Electrode In A Galvanic Cell At Which Oxidation Occurs by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the. The Electrode In A Galvanic Cell At Which Oxidation Occurs.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID1951076 The Electrode In A Galvanic Cell At Which Oxidation Occurs by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the. by definition, the anode of an electrochemical cell is the electrode. The Electrode In A Galvanic Cell At Which Oxidation Occurs.
From www.scienceabc.com
What Are Galvanic Cells? An Oversimplified Explanation » ScienceABC The Electrode In A Galvanic Cell At Which Oxidation Occurs by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode. The name refers to the flow of anions in the. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the. by. The Electrode In A Galvanic Cell At Which Oxidation Occurs.
From www.bigstockphoto.com
Voltaic Galvanic Cell Image & Photo (Free Trial) Bigstock The Electrode In A Galvanic Cell At Which Oxidation Occurs by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag foil). by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is. The Electrode In A Galvanic Cell At Which Oxidation Occurs.
From www.dreamstime.com
Simple Electrochemical or Galvanic Cell. the Daniell Cell. Stock Vector The Electrode In A Galvanic Cell At Which Oxidation Occurs The name refers to the flow of anions in the. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode. by. The Electrode In A Galvanic Cell At Which Oxidation Occurs.
From studybreathings.z21.web.core.windows.net
How Do Electrons Flow In A Galvanic Cell The Electrode In A Galvanic Cell At Which Oxidation Occurs by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag foil). by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the. by definition, the anode of. The Electrode In A Galvanic Cell At Which Oxidation Occurs.
From www.slideserve.com
PPT Galvanic Cells PowerPoint Presentation, free download ID3222668 The Electrode In A Galvanic Cell At Which Oxidation Occurs by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag foil). by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is. The Electrode In A Galvanic Cell At Which Oxidation Occurs.
From employees.csbsju.edu
CHEM 123 GENERAL CHEMISTRY 1 The Electrode In A Galvanic Cell At Which Oxidation Occurs by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag foil). by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the. by definition, the anode of. The Electrode In A Galvanic Cell At Which Oxidation Occurs.
From userdatarheumatics.z21.web.core.windows.net
Simple Cell Diagram Chemistry The Electrode In A Galvanic Cell At Which Oxidation Occurs by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the. The name refers to the flow of anions in the. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction. The Electrode In A Galvanic Cell At Which Oxidation Occurs.
From stock.adobe.com
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and The Electrode In A Galvanic Cell At Which Oxidation Occurs by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the. The name refers to the flow of anions in the. by. The Electrode In A Galvanic Cell At Which Oxidation Occurs.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working The Electrode In A Galvanic Cell At Which Oxidation Occurs by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag foil). The name refers to the flow. The Electrode In A Galvanic Cell At Which Oxidation Occurs.
From www.tes.com
Galvanic Cells, Anode, Cathode, Oxidation, Reduction, Grade 12 The Electrode In A Galvanic Cell At Which Oxidation Occurs The name refers to the flow of anions in the. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag foil). by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs. The Electrode In A Galvanic Cell At Which Oxidation Occurs.
From courses.lumenlearning.com
Galvanic Cells Chemistry The Electrode In A Galvanic Cell At Which Oxidation Occurs by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode. The name refers to the flow of anions in the. by. The Electrode In A Galvanic Cell At Which Oxidation Occurs.
From general.chemistrysteps.com
Galvanic Cells Chemistry Steps The Electrode In A Galvanic Cell At Which Oxidation Occurs by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode. by definition, the anode of an electrochemical cell is the electrode. The Electrode In A Galvanic Cell At Which Oxidation Occurs.
From www.slideserve.com
PPT Voltaic/Galvanic Cells PowerPoint Presentation, free download The Electrode In A Galvanic Cell At Which Oxidation Occurs by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the. The Electrode In A Galvanic Cell At Which Oxidation Occurs.
From www.youtube.com
Galvanic cell or Voltaic cell Definition ,Construction and Working The Electrode In A Galvanic Cell At Which Oxidation Occurs The name refers to the flow of anions in the. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction. The Electrode In A Galvanic Cell At Which Oxidation Occurs.
From chem.libretexts.org
Chapter 19.7 Electrolysis Chemistry LibreTexts The Electrode In A Galvanic Cell At Which Oxidation Occurs by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode. by definition, the. The Electrode In A Galvanic Cell At Which Oxidation Occurs.
From www.jove.com
Voltaic/Galvanic Cells Principle, Components, Cell Notation JoVE The Electrode In A Galvanic Cell At Which Oxidation Occurs by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag foil). by definition, the anode of. The Electrode In A Galvanic Cell At Which Oxidation Occurs.
From chem.libretexts.org
11.1 Galvanic Cells Chemistry LibreTexts The Electrode In A Galvanic Cell At Which Oxidation Occurs by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode. The name refers to. The Electrode In A Galvanic Cell At Which Oxidation Occurs.
From electrochemistryh5t34.blogspot.com
We Love Electrochemistry Galvanic Cell The Electrode In A Galvanic Cell At Which Oxidation Occurs The name refers to the flow of anions in the. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode. by. The Electrode In A Galvanic Cell At Which Oxidation Occurs.
From mungfali.com
Galvanic And Electrolytic Cells The Electrode In A Galvanic Cell At Which Oxidation Occurs by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag foil). The name refers to the flow of anions in the. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs. The Electrode In A Galvanic Cell At Which Oxidation Occurs.
From www.slideserve.com
PPT Galvanic (= voltaic) Cells PowerPoint Presentation, free download The Electrode In A Galvanic Cell At Which Oxidation Occurs by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the. by definition, the anode of an electrochemical cell is the electrode. The Electrode In A Galvanic Cell At Which Oxidation Occurs.
From courses.lumenlearning.com
Galvanic Cells Chemistry The Electrode In A Galvanic Cell At Which Oxidation Occurs by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode. by definition, the. The Electrode In A Galvanic Cell At Which Oxidation Occurs.
From 2012books.lardbucket.org
Electrochemistry The Electrode In A Galvanic Cell At Which Oxidation Occurs by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode. The name refers to the flow of anions in the. by. The Electrode In A Galvanic Cell At Which Oxidation Occurs.
From www.vecteezy.com
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and The Electrode In A Galvanic Cell At Which Oxidation Occurs The name refers to the flow of anions in the. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode. by. The Electrode In A Galvanic Cell At Which Oxidation Occurs.
From www.slideserve.com
PPT Galvanic Cells PowerPoint Presentation, free download ID3222668 The Electrode In A Galvanic Cell At Which Oxidation Occurs The name refers to the flow of anions in the. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode. by. The Electrode In A Galvanic Cell At Which Oxidation Occurs.
From chem.libretexts.org
1 Electrochemical Cells (Experiment) Chemistry LibreTexts The Electrode In A Galvanic Cell At Which Oxidation Occurs by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode. The name refers to. The Electrode In A Galvanic Cell At Which Oxidation Occurs.
From stock.adobe.com
Vector scientific illustration of the electrolysis processes. Set of The Electrode In A Galvanic Cell At Which Oxidation Occurs by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the. The Electrode In A Galvanic Cell At Which Oxidation Occurs.
From thechemistrynotes.com
Galvanic Cell (Voltaic Cell) Definition, Working Principle The Electrode In A Galvanic Cell At Which Oxidation Occurs by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag foil). by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is. The Electrode In A Galvanic Cell At Which Oxidation Occurs.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts The Electrode In A Galvanic Cell At Which Oxidation Occurs The name refers to the flow of anions in the. by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the electrode where reduction occurs (the ag foil). by definition, the anode of an electrochemical cell is the electrode at which oxidation occurs. The Electrode In A Galvanic Cell At Which Oxidation Occurs.