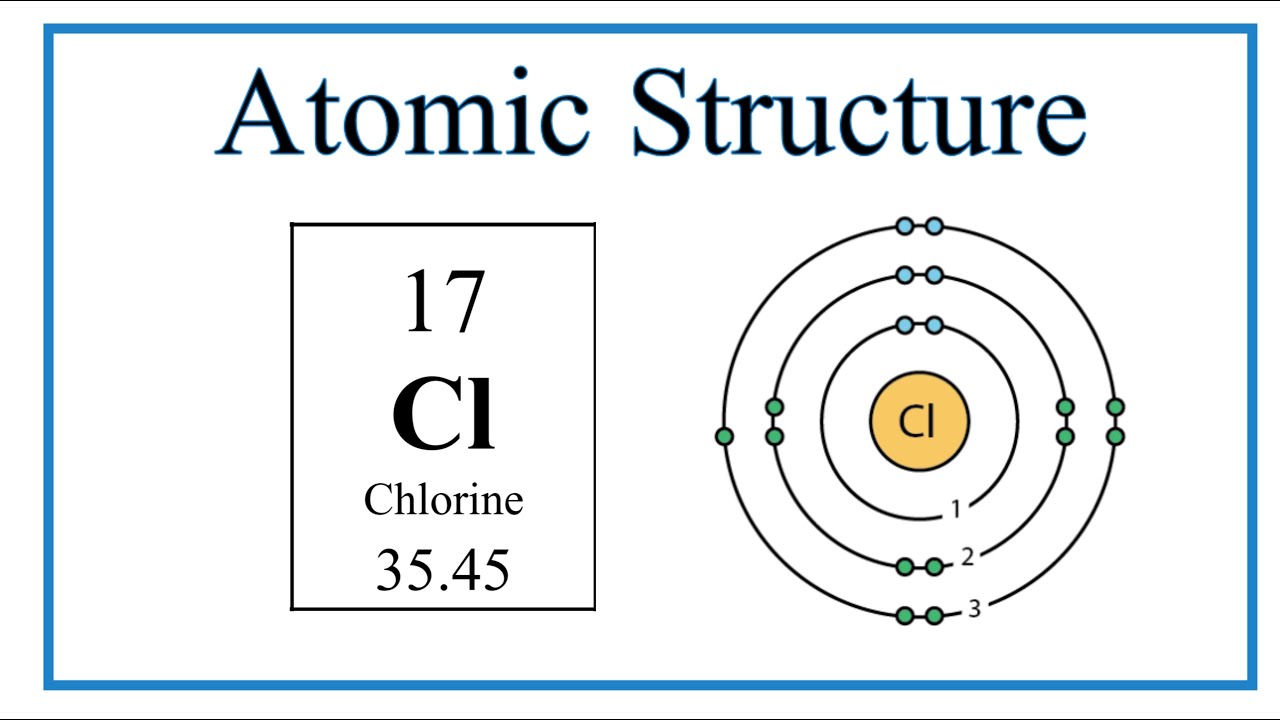
Chlorine-37 Protons Neutrons Electrons . Only two chlorine isotopes exist. Chlorine atom have 17 protons because. How many protons neutrons electrons does a chlorine molecule? Describe the locations, charges, and masses of the three main subatomic particles. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes with 15 to 23 neutrons. The following table shows the atomic nuclei that are isotonic (same neutron number n = 20) and isobaric (same nucleon. Chlorine has an atomic number of 17. Only two chlorine isotopes exist in. Trace amounts of radioactive 36 cl exist in the environment, in a ratio of about 7×10 −13 to 1 with stable isotopes. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes having 15 to 23 neutrons. Determine the number of protons by identifying the atomic number for chlorine on the periodic table. Determine the number of protons and electrons.
from www.youtube.com
How many protons neutrons electrons does a chlorine molecule? Describe the locations, charges, and masses of the three main subatomic particles. The following table shows the atomic nuclei that are isotonic (same neutron number n = 20) and isobaric (same nucleon. Determine the number of protons and electrons. Only two chlorine isotopes exist. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes with 15 to 23 neutrons. Only two chlorine isotopes exist in. Chlorine atom have 17 protons because. Trace amounts of radioactive 36 cl exist in the environment, in a ratio of about 7×10 −13 to 1 with stable isotopes. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes having 15 to 23 neutrons.
Atomic Structure (Bohr Model) for Chlorine (Cl) YouTube
Chlorine-37 Protons Neutrons Electrons Chlorine atom have 17 protons because. Chlorine atom have 17 protons because. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes with 15 to 23 neutrons. Determine the number of protons and electrons. Only two chlorine isotopes exist. Only two chlorine isotopes exist in. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes having 15 to 23 neutrons. Determine the number of protons by identifying the atomic number for chlorine on the periodic table. Chlorine has an atomic number of 17. Trace amounts of radioactive 36 cl exist in the environment, in a ratio of about 7×10 −13 to 1 with stable isotopes. The following table shows the atomic nuclei that are isotonic (same neutron number n = 20) and isobaric (same nucleon. Describe the locations, charges, and masses of the three main subatomic particles. How many protons neutrons electrons does a chlorine molecule?
From www.slideserve.com
PPT How many protons, neutrons, and electrons are there in an atom of Chlorine-37 Protons Neutrons Electrons All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes with 15 to 23 neutrons. Chlorine has an atomic number of 17. Describe the locations, charges, and masses of the three main subatomic particles. Determine the number of protons by identifying the atomic number for chlorine on the periodic table. Only two chlorine isotopes exist in. The. Chlorine-37 Protons Neutrons Electrons.
From sciencenotes.org
Chlorine Facts Chlorine-37 Protons Neutrons Electrons Describe the locations, charges, and masses of the three main subatomic particles. Determine the number of protons and electrons. Only two chlorine isotopes exist. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes with 15 to 23 neutrons. Chlorine atom have 17 protons because. Trace amounts of radioactive 36 cl exist in the environment, in a. Chlorine-37 Protons Neutrons Electrons.
From utedzz.blogspot.com
Periodic Table Chlorine Atomic Number Periodic Table Timeline Chlorine-37 Protons Neutrons Electrons Trace amounts of radioactive 36 cl exist in the environment, in a ratio of about 7×10 −13 to 1 with stable isotopes. Determine the number of protons by identifying the atomic number for chlorine on the periodic table. Only two chlorine isotopes exist. Describe the locations, charges, and masses of the three main subatomic particles. All atoms of chlorine (cl). Chlorine-37 Protons Neutrons Electrons.
From elchoroukhost.net
Chlorine Periodic Table Protons Neutrons Electrons Elcho Table Chlorine-37 Protons Neutrons Electrons How many protons neutrons electrons does a chlorine molecule? All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes having 15 to 23 neutrons. Determine the number of protons by identifying the atomic number for chlorine on the periodic table. Chlorine has an atomic number of 17. Describe the locations, charges, and masses of the three main. Chlorine-37 Protons Neutrons Electrons.
From www.numerade.com
SOLVED How many protons; neutrons and electrons are there in a neutral Chlorine-37 Protons Neutrons Electrons Trace amounts of radioactive 36 cl exist in the environment, in a ratio of about 7×10 −13 to 1 with stable isotopes. Determine the number of protons and electrons. Describe the locations, charges, and masses of the three main subatomic particles. Only two chlorine isotopes exist in. The following table shows the atomic nuclei that are isotonic (same neutron number. Chlorine-37 Protons Neutrons Electrons.
From brokeasshome.com
Chlorine Periodic Table Protons Neutrons Electrons Chlorine-37 Protons Neutrons Electrons Only two chlorine isotopes exist in. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes having 15 to 23 neutrons. How many protons neutrons electrons does a chlorine molecule? Determine the number of protons and electrons. Only two chlorine isotopes exist. Trace amounts of radioactive 36 cl exist in the environment, in a ratio of about. Chlorine-37 Protons Neutrons Electrons.
From ar.inspiredpencil.com
Chlorine Protons Neutrons Electrons Chlorine-37 Protons Neutrons Electrons Chlorine has an atomic number of 17. Determine the number of protons and electrons. How many protons neutrons electrons does a chlorine molecule? Determine the number of protons by identifying the atomic number for chlorine on the periodic table. Only two chlorine isotopes exist in. The following table shows the atomic nuclei that are isotonic (same neutron number n =. Chlorine-37 Protons Neutrons Electrons.
From brokeasshome.com
Chlorine Periodic Table Protons Neutrons Electrons Chlorine-37 Protons Neutrons Electrons The following table shows the atomic nuclei that are isotonic (same neutron number n = 20) and isobaric (same nucleon. Trace amounts of radioactive 36 cl exist in the environment, in a ratio of about 7×10 −13 to 1 with stable isotopes. Determine the number of protons by identifying the atomic number for chlorine on the periodic table. All atoms. Chlorine-37 Protons Neutrons Electrons.
From newtondesk.com
Chlorine Cl (Element 17) of Periodic Table Chlorine-37 Protons Neutrons Electrons Describe the locations, charges, and masses of the three main subatomic particles. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes with 15 to 23 neutrons. Chlorine atom have 17 protons because. Only two chlorine isotopes exist in. Determine the number of protons and electrons. Only two chlorine isotopes exist. How many protons neutrons electrons does. Chlorine-37 Protons Neutrons Electrons.
From valenceelectrons.com
How to find the Protons, Neutrons and Electrons for Chlorine? Chlorine-37 Protons Neutrons Electrons Determine the number of protons and electrons. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes having 15 to 23 neutrons. Only two chlorine isotopes exist. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes with 15 to 23 neutrons. Chlorine has an atomic number of 17. Describe the locations, charges, and. Chlorine-37 Protons Neutrons Electrons.
From www.numerade.com
SOLVED 'This image shows............... This image shows points 37 1 Chlorine-37 Protons Neutrons Electrons The following table shows the atomic nuclei that are isotonic (same neutron number n = 20) and isobaric (same nucleon. Chlorine has an atomic number of 17. Only two chlorine isotopes exist. Determine the number of protons and electrons. Describe the locations, charges, and masses of the three main subatomic particles. Only two chlorine isotopes exist in. All atoms of. Chlorine-37 Protons Neutrons Electrons.
From www.alamy.com
Chlorine (Cl). Diagram of the electron configuration of an atom of Chlorine-37 Protons Neutrons Electrons All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes with 15 to 23 neutrons. The following table shows the atomic nuclei that are isotonic (same neutron number n = 20) and isobaric (same nucleon. Trace amounts of radioactive 36 cl exist in the environment, in a ratio of about 7×10 −13 to 1 with stable isotopes.. Chlorine-37 Protons Neutrons Electrons.
From ar.inspiredpencil.com
Chlorine Protons Neutrons Electrons Chlorine-37 Protons Neutrons Electrons Only two chlorine isotopes exist in. Determine the number of protons and electrons. Only two chlorine isotopes exist. Chlorine atom have 17 protons because. How many protons neutrons electrons does a chlorine molecule? Determine the number of protons by identifying the atomic number for chlorine on the periodic table. Describe the locations, charges, and masses of the three main subatomic. Chlorine-37 Protons Neutrons Electrons.
From www.youtube.com
Atomic Structure (Bohr Model) for Chlorine (Cl) YouTube Chlorine-37 Protons Neutrons Electrons How many protons neutrons electrons does a chlorine molecule? Only two chlorine isotopes exist in. Chlorine has an atomic number of 17. Trace amounts of radioactive 36 cl exist in the environment, in a ratio of about 7×10 −13 to 1 with stable isotopes. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes with 15 to. Chlorine-37 Protons Neutrons Electrons.
From ar.inspiredpencil.com
Chlorine Protons Neutrons Electrons Chlorine-37 Protons Neutrons Electrons Only two chlorine isotopes exist. Chlorine atom have 17 protons because. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes with 15 to 23 neutrons. Determine the number of protons and electrons. Trace amounts of radioactive 36 cl exist in the environment, in a ratio of about 7×10 −13 to 1 with stable isotopes. Describe the. Chlorine-37 Protons Neutrons Electrons.
From material-properties.org
Chlorine Protons Neutrons Electrons Electron Configuration Chlorine-37 Protons Neutrons Electrons Describe the locations, charges, and masses of the three main subatomic particles. Trace amounts of radioactive 36 cl exist in the environment, in a ratio of about 7×10 −13 to 1 with stable isotopes. Determine the number of protons and electrons. Chlorine atom have 17 protons because. Only two chlorine isotopes exist in. How many protons neutrons electrons does a. Chlorine-37 Protons Neutrons Electrons.
From www.dreamstime.com
Diagram Representation Of The Element Chlorine Stock Illustration Chlorine-37 Protons Neutrons Electrons Determine the number of protons and electrons. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes having 15 to 23 neutrons. The following table shows the atomic nuclei that are isotonic (same neutron number n = 20) and isobaric (same nucleon. Only two chlorine isotopes exist. All atoms of chlorine (cl) have 17 protons, but there. Chlorine-37 Protons Neutrons Electrons.
From www.youtube.com
How to find the Number of Protons, Electrons, Neutrons for Chlorine (Cl Chlorine-37 Protons Neutrons Electrons Determine the number of protons by identifying the atomic number for chlorine on the periodic table. The following table shows the atomic nuclei that are isotonic (same neutron number n = 20) and isobaric (same nucleon. How many protons neutrons electrons does a chlorine molecule? All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes having 15. Chlorine-37 Protons Neutrons Electrons.
From ar.inspiredpencil.com
Chlorine Protons Neutrons Electrons Chlorine-37 Protons Neutrons Electrons Only two chlorine isotopes exist in. Determine the number of protons by identifying the atomic number for chlorine on the periodic table. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes with 15 to 23 neutrons. Chlorine atom have 17 protons because. Describe the locations, charges, and masses of the three main subatomic particles. Determine the. Chlorine-37 Protons Neutrons Electrons.
From chamotgallery.com
How many protons, neutrons and electrons does chlorine have? (2023) Chlorine-37 Protons Neutrons Electrons Chlorine has an atomic number of 17. Trace amounts of radioactive 36 cl exist in the environment, in a ratio of about 7×10 −13 to 1 with stable isotopes. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes having 15 to 23 neutrons. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes. Chlorine-37 Protons Neutrons Electrons.
From www.sciencephoto.com
Isotopes of chlorine, illustration Stock Image C028/6461 Science Chlorine-37 Protons Neutrons Electrons Determine the number of protons and electrons. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes having 15 to 23 neutrons. Only two chlorine isotopes exist. Trace amounts of radioactive 36 cl exist in the environment, in a ratio of about 7×10 −13 to 1 with stable isotopes. Chlorine has an atomic number of 17. Chlorine. Chlorine-37 Protons Neutrons Electrons.
From ar.inspiredpencil.com
Chlorine Protons Neutrons Electrons Chlorine-37 Protons Neutrons Electrons The following table shows the atomic nuclei that are isotonic (same neutron number n = 20) and isobaric (same nucleon. Chlorine atom have 17 protons because. How many protons neutrons electrons does a chlorine molecule? All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes having 15 to 23 neutrons. Determine the number of protons by identifying. Chlorine-37 Protons Neutrons Electrons.
From ar.inspiredpencil.com
Chlorine Bohr Model Chlorine-37 Protons Neutrons Electrons Trace amounts of radioactive 36 cl exist in the environment, in a ratio of about 7×10 −13 to 1 with stable isotopes. The following table shows the atomic nuclei that are isotonic (same neutron number n = 20) and isobaric (same nucleon. Only two chlorine isotopes exist in. All atoms of chlorine (cl) have 17 protons, but there are chlorine. Chlorine-37 Protons Neutrons Electrons.
From brokeasshome.com
Chlorine Periodic Table Protons Neutrons Electrons Chlorine-37 Protons Neutrons Electrons Only two chlorine isotopes exist. Chlorine has an atomic number of 17. Trace amounts of radioactive 36 cl exist in the environment, in a ratio of about 7×10 −13 to 1 with stable isotopes. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes having 15 to 23 neutrons. All atoms of chlorine (cl) have 17 protons,. Chlorine-37 Protons Neutrons Electrons.
From www.teachoo.com
Isotopes and Isobars Definition, Uses and Difference Teachoo Chlorine-37 Protons Neutrons Electrons Determine the number of protons and electrons. Determine the number of protons by identifying the atomic number for chlorine on the periodic table. Only two chlorine isotopes exist. Chlorine atom have 17 protons because. Describe the locations, charges, and masses of the three main subatomic particles. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes with. Chlorine-37 Protons Neutrons Electrons.
From www.slideserve.com
PPT Relative atomic mass PowerPoint Presentation ID5933036 Chlorine-37 Protons Neutrons Electrons The following table shows the atomic nuclei that are isotonic (same neutron number n = 20) and isobaric (same nucleon. How many protons neutrons electrons does a chlorine molecule? Chlorine has an atomic number of 17. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes having 15 to 23 neutrons. All atoms of chlorine (cl) have. Chlorine-37 Protons Neutrons Electrons.
From elchoroukhost.net
Chlorine Periodic Table Protons Neutrons Electrons Elcho Table Chlorine-37 Protons Neutrons Electrons Determine the number of protons by identifying the atomic number for chlorine on the periodic table. Describe the locations, charges, and masses of the three main subatomic particles. Only two chlorine isotopes exist in. Determine the number of protons and electrons. Chlorine atom have 17 protons because. Only two chlorine isotopes exist. How many protons neutrons electrons does a chlorine. Chlorine-37 Protons Neutrons Electrons.
From brokeasshome.com
Chlorine Periodic Table Protons Neutrons Electrons Chlorine-37 Protons Neutrons Electrons Determine the number of protons by identifying the atomic number for chlorine on the periodic table. Only two chlorine isotopes exist in. Describe the locations, charges, and masses of the three main subatomic particles. The following table shows the atomic nuclei that are isotonic (same neutron number n = 20) and isobaric (same nucleon. Determine the number of protons and. Chlorine-37 Protons Neutrons Electrons.
From www.sciencephoto.com
Chlorine electron configuration Stock Image C029/5025 Science Photo Chlorine-37 Protons Neutrons Electrons Only two chlorine isotopes exist. Only two chlorine isotopes exist in. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes with 15 to 23 neutrons. Determine the number of protons and electrons. Trace amounts of radioactive 36 cl exist in the environment, in a ratio of about 7×10 −13 to 1 with stable isotopes. Chlorine has. Chlorine-37 Protons Neutrons Electrons.
From brokeasshome.com
Chlorine Periodic Table Protons Neutrons Electrons Chlorine-37 Protons Neutrons Electrons Determine the number of protons and electrons. Only two chlorine isotopes exist. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes having 15 to 23 neutrons. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes with 15 to 23 neutrons. How many protons neutrons electrons does a chlorine molecule? Only two chlorine. Chlorine-37 Protons Neutrons Electrons.
From www.shutterstock.com
Atom Chlorine This Diagram Shows Electron Stock Vector 328668782 Chlorine-37 Protons Neutrons Electrons How many protons neutrons electrons does a chlorine molecule? Chlorine has an atomic number of 17. Determine the number of protons by identifying the atomic number for chlorine on the periodic table. Only two chlorine isotopes exist. Only two chlorine isotopes exist in. Describe the locations, charges, and masses of the three main subatomic particles. Chlorine atom have 17 protons. Chlorine-37 Protons Neutrons Electrons.
From www.slideserve.com
PPT How many protons, neutrons, and electrons are there in an atom of Chlorine-37 Protons Neutrons Electrons Chlorine atom have 17 protons because. Determine the number of protons by identifying the atomic number for chlorine on the periodic table. The following table shows the atomic nuclei that are isotonic (same neutron number n = 20) and isobaric (same nucleon. Chlorine has an atomic number of 17. All atoms of chlorine (cl) have 17 protons, but there are. Chlorine-37 Protons Neutrons Electrons.
From elchoroukhost.net
Chlorine Periodic Table Protons Neutrons Electrons Elcho Table Chlorine-37 Protons Neutrons Electrons Only two chlorine isotopes exist in. Chlorine atom have 17 protons because. Chlorine has an atomic number of 17. Trace amounts of radioactive 36 cl exist in the environment, in a ratio of about 7×10 −13 to 1 with stable isotopes. Determine the number of protons and electrons. How many protons neutrons electrons does a chlorine molecule? All atoms of. Chlorine-37 Protons Neutrons Electrons.
From www.slideserve.com
PPT electron cloud PowerPoint Presentation, free download ID5937173 Chlorine-37 Protons Neutrons Electrons Chlorine has an atomic number of 17. How many protons neutrons electrons does a chlorine molecule? Only two chlorine isotopes exist. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes having 15 to 23 neutrons. Only two chlorine isotopes exist in. Trace amounts of radioactive 36 cl exist in the environment, in a ratio of about. Chlorine-37 Protons Neutrons Electrons.
From stock.adobe.com
Chlorine atomic structure has atomic number, atomic mass, electron Chlorine-37 Protons Neutrons Electrons The following table shows the atomic nuclei that are isotonic (same neutron number n = 20) and isobaric (same nucleon. Describe the locations, charges, and masses of the three main subatomic particles. Determine the number of protons by identifying the atomic number for chlorine on the periodic table. Only two chlorine isotopes exist. Only two chlorine isotopes exist in. All. Chlorine-37 Protons Neutrons Electrons.