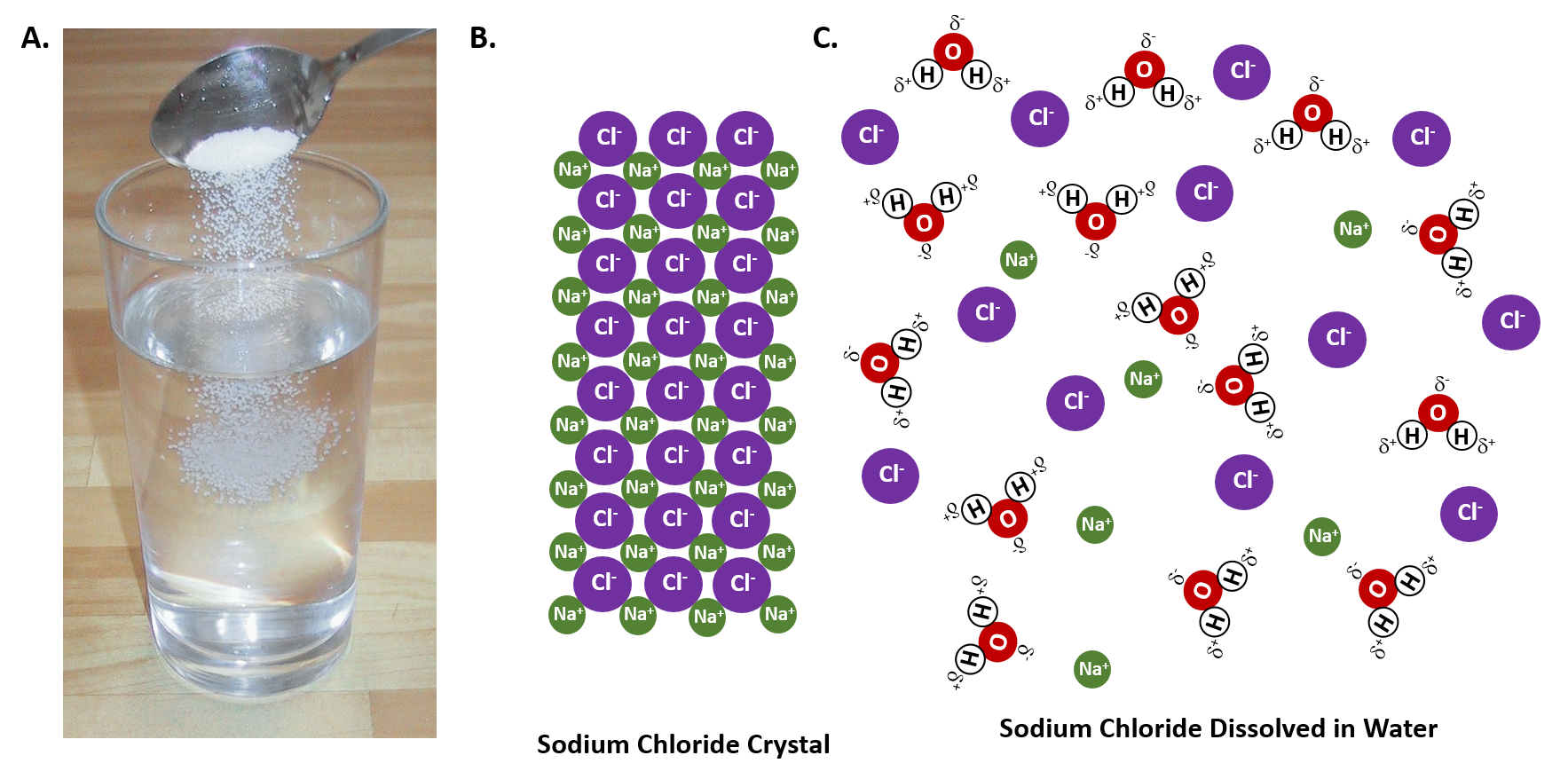
Do Salt Dissolve In Boiling Water . The temperature needed to boil will increase by about 0.5 c for every 58 grams of dissolved salt per kilogram of water. I have often added salt to boiling water, and i used to expect it to stop boiling momentarily, to catch up to the new boiling point. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium. If you add salt to water, you raise the boiling point, or the temperature at which water boils. The boiling point of water increases when salt is dissolved in it. This phenomenon is known as boiling point. Similarly, you also lower its freezing point. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water
from www.wou.edu
Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water If you add salt to water, you raise the boiling point, or the temperature at which water boils. The temperature needed to boil will increase by about 0.5 c for every 58 grams of dissolved salt per kilogram of water. This phenomenon is known as boiling point. I have often added salt to boiling water, and i used to expect it to stop boiling momentarily, to catch up to the new boiling point. The boiling point of water increases when salt is dissolved in it. Similarly, you also lower its freezing point.
CH150 Chapter 7 Solutions Chemistry
Do Salt Dissolve In Boiling Water If you add salt to water, you raise the boiling point, or the temperature at which water boils. The temperature needed to boil will increase by about 0.5 c for every 58 grams of dissolved salt per kilogram of water. If you add salt to water, you raise the boiling point, or the temperature at which water boils. The boiling point of water increases when salt is dissolved in it. This phenomenon is known as boiling point. I have often added salt to boiling water, and i used to expect it to stop boiling momentarily, to catch up to the new boiling point. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium. Similarly, you also lower its freezing point.
From materialcampusdecurion.z5.web.core.windows.net
Separation Of Salt And Water Do Salt Dissolve In Boiling Water This phenomenon is known as boiling point. The temperature needed to boil will increase by about 0.5 c for every 58 grams of dissolved salt per kilogram of water. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium. I have often added. Do Salt Dissolve In Boiling Water.
From ar.inspiredpencil.com
Salt And Boiling Water Do Salt Dissolve In Boiling Water I have often added salt to boiling water, and i used to expect it to stop boiling momentarily, to catch up to the new boiling point. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water The temperature needed to boil will increase by about 0.5 c for every. Do Salt Dissolve In Boiling Water.
From schematiclistpact101.z22.web.core.windows.net
Salt Dissolving In Water Diagram Do Salt Dissolve In Boiling Water If you add salt to water, you raise the boiling point, or the temperature at which water boils. I have often added salt to boiling water, and i used to expect it to stop boiling momentarily, to catch up to the new boiling point. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions,. Do Salt Dissolve In Boiling Water.
From www.wikihow.com
How to Dissolve Salt in Water 9 Steps (with Pictures) wikiHow Do Salt Dissolve In Boiling Water Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium. If you add salt to water, you raise the boiling point, or the temperature at which water boils. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then. Do Salt Dissolve In Boiling Water.
From www.vectorstock.com
Salt boiling water process isolated on white Vector Image Do Salt Dissolve In Boiling Water If you add salt to water, you raise the boiling point, or the temperature at which water boils. The boiling point of water increases when salt is dissolved in it. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium. I have often. Do Salt Dissolve In Boiling Water.
From www.slideserve.com
PPT Chapter 8 SOLUTIONS PowerPoint Presentation, free download ID Do Salt Dissolve In Boiling Water Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium. If you add salt to water, you raise the boiling point, or the temperature at which water boils. I have often added salt to boiling water, and i used to expect it to. Do Salt Dissolve In Boiling Water.
From www.science-sparks.com
Which Solids Dissolve In Water Cool Science for Kids Do Salt Dissolve In Boiling Water A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water This phenomenon is known as boiling point. The boiling point of water increases when salt is dissolved in it. I have often added salt to boiling water, and i used to expect it to stop boiling momentarily, to catch. Do Salt Dissolve In Boiling Water.
From energyeducation.ca
Solute Energy Education Do Salt Dissolve In Boiling Water A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water If you add salt to water, you raise the boiling point, or the temperature at which water boils. Similarly, you also lower its freezing point. The boiling point of water increases when salt is dissolved in it. Water can. Do Salt Dissolve In Boiling Water.
From www.youtube.com
Dissolving Salt in Water YouTube Do Salt Dissolve In Boiling Water The boiling point of water increases when salt is dissolved in it. If you add salt to water, you raise the boiling point, or the temperature at which water boils. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium. Similarly, you also. Do Salt Dissolve In Boiling Water.
From www.labkafe.com
Solubility of Salts ‒ Why Common Salts are So Soluble in Water Labkafe Do Salt Dissolve In Boiling Water Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium. I have often added salt to boiling water, and i used to expect it to stop boiling momentarily, to catch up to the new boiling point. The temperature needed to boil will increase. Do Salt Dissolve In Boiling Water.
From www.dreamstime.com
Add Salt To Boiling Water in a Metal Pan Closeup Stock Image Image Do Salt Dissolve In Boiling Water Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water This phenomenon is known as boiling point. Similarly, you also lower its. Do Salt Dissolve In Boiling Water.
From www.youtube.com
How Salt Dissolves in Water? YouTube Do Salt Dissolve In Boiling Water The boiling point of water increases when salt is dissolved in it. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water Similarly, you also lower its freezing point. This phenomenon is known as boiling point. I have often added salt to boiling water, and i used to expect. Do Salt Dissolve In Boiling Water.
From bettertogether.org
Is Salt Dissolving In Water A Chemical Change Better Together Do Salt Dissolve In Boiling Water The boiling point of water increases when salt is dissolved in it. Similarly, you also lower its freezing point. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium. If you add salt to water, you raise the boiling point, or the temperature. Do Salt Dissolve In Boiling Water.
From www.thoughtco.com
Dissolving Salt in Water Chemical or Physical Change? Do Salt Dissolve In Boiling Water If you add salt to water, you raise the boiling point, or the temperature at which water boils. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of. Do Salt Dissolve In Boiling Water.
From www.youtube.com
Salt dissolves in water Is this a chemical or physical change? YouTube Do Salt Dissolve In Boiling Water If you add salt to water, you raise the boiling point, or the temperature at which water boils. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium. I have often added salt to boiling water, and i used to expect it to. Do Salt Dissolve In Boiling Water.
From www.wou.edu
CH150 Chapter 7 Solutions Chemistry Do Salt Dissolve In Boiling Water The boiling point of water increases when salt is dissolved in it. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium. The temperature needed to boil will increase by about 0.5 c for every 58 grams of dissolved salt per kilogram of. Do Salt Dissolve In Boiling Water.
From www.slideserve.com
PPT Chemical Reactions Chapter 7 PowerPoint Presentation, free Do Salt Dissolve In Boiling Water Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium. Similarly, you also lower its freezing point. The boiling point of water increases when salt is dissolved in it. I have often added salt to boiling water, and i used to expect it. Do Salt Dissolve In Boiling Water.
From fphoto.photoshelter.com
science chemistry experiment solubility Fundamental Photographs The Do Salt Dissolve In Boiling Water Similarly, you also lower its freezing point. The boiling point of water increases when salt is dissolved in it. If you add salt to water, you raise the boiling point, or the temperature at which water boils. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water Water can. Do Salt Dissolve In Boiling Water.
From www.vectorstock.com
How does sodium chloride nacl dissolve in water Vector Image Do Salt Dissolve In Boiling Water Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium. I have often added salt to boiling water, and i used to expect it to stop boiling momentarily, to catch up to the new boiling point. The temperature needed to boil will increase. Do Salt Dissolve In Boiling Water.
From lessonlangdonodeon.z21.web.core.windows.net
Boiling Salt Water To Drink Do Salt Dissolve In Boiling Water I have often added salt to boiling water, and i used to expect it to stop boiling momentarily, to catch up to the new boiling point. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium. A machine learning model has revealed how. Do Salt Dissolve In Boiling Water.
From schematicfixtrysted.z22.web.core.windows.net
Diagram Of Salt Dissolving In Water Do Salt Dissolve In Boiling Water If you add salt to water, you raise the boiling point, or the temperature at which water boils. This phenomenon is known as boiling point. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water I have often added salt to boiling water, and i used to expect it. Do Salt Dissolve In Boiling Water.
From www.wikihow.com
How to Dissolve Salt in Water 9 Steps (with Pictures) wikiHow Do Salt Dissolve In Boiling Water If you add salt to water, you raise the boiling point, or the temperature at which water boils. The temperature needed to boil will increase by about 0.5 c for every 58 grams of dissolved salt per kilogram of water. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part. Do Salt Dissolve In Boiling Water.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Do Salt Dissolve In Boiling Water If you add salt to water, you raise the boiling point, or the temperature at which water boils. The temperature needed to boil will increase by about 0.5 c for every 58 grams of dissolved salt per kilogram of water. This phenomenon is known as boiling point. Similarly, you also lower its freezing point. The boiling point of water increases. Do Salt Dissolve In Boiling Water.
From www.wikihow.com
How to Dissolve Salt in Water 9 Steps (with Pictures) wikiHow Do Salt Dissolve In Boiling Water I have often added salt to boiling water, and i used to expect it to stop boiling momentarily, to catch up to the new boiling point. The boiling point of water increases when salt is dissolved in it. Similarly, you also lower its freezing point. This phenomenon is known as boiling point. Water can dissolve salt because the positive part. Do Salt Dissolve In Boiling Water.
From www.bbc.co.uk
Distillation BBC Bitesize Do Salt Dissolve In Boiling Water I have often added salt to boiling water, and i used to expect it to stop boiling momentarily, to catch up to the new boiling point. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water The boiling point of water increases when salt is dissolved in it. The. Do Salt Dissolve In Boiling Water.
From www.dreamstime.com
Add Salt To Boiling Water in a Metal Pan Closeup Stock Image Image Do Salt Dissolve In Boiling Water If you add salt to water, you raise the boiling point, or the temperature at which water boils. I have often added salt to boiling water, and i used to expect it to stop boiling momentarily, to catch up to the new boiling point. The temperature needed to boil will increase by about 0.5 c for every 58 grams of. Do Salt Dissolve In Boiling Water.
From www.youtube.com
Essential Chemistry Dissolving Salt in Water YouTube Do Salt Dissolve In Boiling Water This phenomenon is known as boiling point. Similarly, you also lower its freezing point. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water The boiling point of water increases when salt is dissolved in it. I have often added salt to boiling water, and i used to expect. Do Salt Dissolve In Boiling Water.
From www.slideserve.com
PPT Chapter 13 Solutions PowerPoint Presentation, free download ID Do Salt Dissolve In Boiling Water The boiling point of water increases when salt is dissolved in it. If you add salt to water, you raise the boiling point, or the temperature at which water boils. Similarly, you also lower its freezing point. The temperature needed to boil will increase by about 0.5 c for every 58 grams of dissolved salt per kilogram of water. Water. Do Salt Dissolve In Boiling Water.
From eduvik.in
Matter in Our Surroundings Class 9 Notes Science Chapter 1 Eduvik Do Salt Dissolve In Boiling Water This phenomenon is known as boiling point. Similarly, you also lower its freezing point. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water The boiling point of water increases when salt is dissolved in it. I have often added salt to boiling water, and i used to expect. Do Salt Dissolve In Boiling Water.
From www.slideserve.com
PPT BASIC’S OF SOLUTION CHEMISTRY PowerPoint Presentation, free Do Salt Dissolve In Boiling Water Similarly, you also lower its freezing point. I have often added salt to boiling water, and i used to expect it to stop boiling momentarily, to catch up to the new boiling point. This phenomenon is known as boiling point. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in. Do Salt Dissolve In Boiling Water.
From www.youtube.com
Chemistry Animation Salt in Boiling Water.wmv YouTube Do Salt Dissolve In Boiling Water This phenomenon is known as boiling point. If you add salt to water, you raise the boiling point, or the temperature at which water boils. Similarly, you also lower its freezing point. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium. I. Do Salt Dissolve In Boiling Water.
From www.cookist.com
Why Do Some People Add Salt To Boiling Water? Do Salt Dissolve In Boiling Water I have often added salt to boiling water, and i used to expect it to stop boiling momentarily, to catch up to the new boiling point. This phenomenon is known as boiling point. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium.. Do Salt Dissolve In Boiling Water.
From manualwiringbrancher.z14.web.core.windows.net
Diagram Of Salt Dissolving In Water Do Salt Dissolve In Boiling Water A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water The temperature needed to boil will increase by about 0.5 c for every 58 grams of dissolved salt per kilogram of water. This phenomenon is known as boiling point. The boiling point of water increases when salt is dissolved. Do Salt Dissolve In Boiling Water.
From www.thoughtco.com
Why Adding Salt to Water Increases the Boiling Point Do Salt Dissolve In Boiling Water The boiling point of water increases when salt is dissolved in it. If you add salt to water, you raise the boiling point, or the temperature at which water boils. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium. Similarly, you also. Do Salt Dissolve In Boiling Water.
From www.wikihow.com
How to Dissolve Salt in Water 9 Steps (with Pictures) wikiHow Do Salt Dissolve In Boiling Water Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium. The temperature needed to boil will increase by about 0.5 c for every 58 grams of dissolved salt per kilogram of water. The boiling point of water increases when salt is dissolved in. Do Salt Dissolve In Boiling Water.