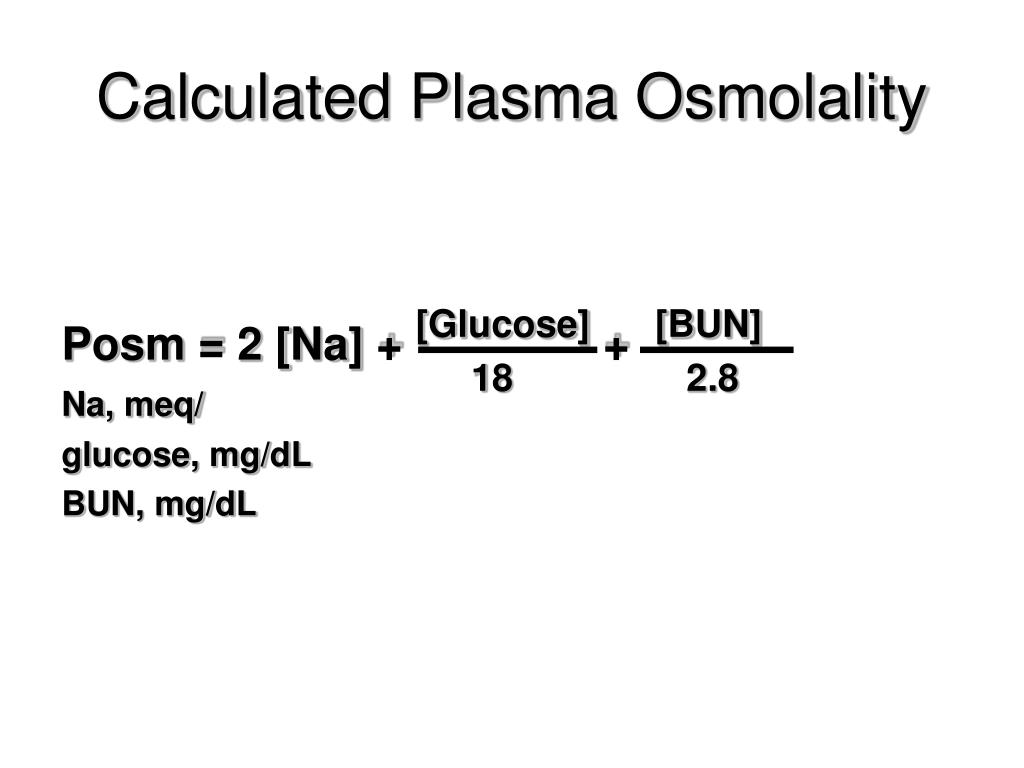
How To Calculate Theoretical Osmolality . Convert that number to osmoles per liter and add the osmolarity of each. An osmole (osmol) is 1 mol of particles that. Osmolarity cannot be measured but is calculated theoretically from the experimentally measured value of osmolality. Osmolarity of a solution is a theoretical quantity expressed in osmoles per l (osmol per l) of a solution and is widely used in clinical practice. To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. Osmolality is milliosmoles of solutes per one kilogram (or liter) of water of solution (plasma) and is calculated by osmolarity divided to. The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. You multiply the molarity by the number of osmoles that each solute produces.
from exonhkusy.blob.core.windows.net
Osmolality is milliosmoles of solutes per one kilogram (or liter) of water of solution (plasma) and is calculated by osmolarity divided to. Convert that number to osmoles per liter and add the osmolarity of each. You multiply the molarity by the number of osmoles that each solute produces. Osmolarity cannot be measured but is calculated theoretically from the experimentally measured value of osmolality. To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. An osmole (osmol) is 1 mol of particles that. The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. Osmolarity of a solution is a theoretical quantity expressed in osmoles per l (osmol per l) of a solution and is widely used in clinical practice.
How To Calculate Osmolality Of Glucose at Robert Villegas blog
How To Calculate Theoretical Osmolality The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. Osmolality is milliosmoles of solutes per one kilogram (or liter) of water of solution (plasma) and is calculated by osmolarity divided to. Convert that number to osmoles per liter and add the osmolarity of each. Osmolarity of a solution is a theoretical quantity expressed in osmoles per l (osmol per l) of a solution and is widely used in clinical practice. You multiply the molarity by the number of osmoles that each solute produces. Osmolarity cannot be measured but is calculated theoretically from the experimentally measured value of osmolality. An osmole (osmol) is 1 mol of particles that. To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma.
From ojklmexhbr.blogspot.com
How To Calculate Serum Osmolality In this study, we compared the How To Calculate Theoretical Osmolality Osmolality is milliosmoles of solutes per one kilogram (or liter) of water of solution (plasma) and is calculated by osmolarity divided to. To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. You multiply the molarity by the number of osmoles that each solute produces. The salts of sodium (choloride and. How To Calculate Theoretical Osmolality.
From www.youtube.com
How to solve osmolarity calculation problems 4 YouTube How To Calculate Theoretical Osmolality To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. Convert that number to osmoles per liter and add the osmolarity of each. Osmolarity cannot be measured but is calculated theoretically from the experimentally measured value of osmolality. An osmole (osmol) is 1 mol of particles that. Osmolality is milliosmoles of. How To Calculate Theoretical Osmolality.
From brionysylvie.blogspot.com
10+ Urine Anion Gap Calculator BrionySylvie How To Calculate Theoretical Osmolality The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. Osmolarity of a solution is a theoretical quantity expressed in osmoles per l (osmol per l) of a solution and is widely used in clinical practice. An osmole (osmol) is 1 mol of particles that. You multiply the molarity by the. How To Calculate Theoretical Osmolality.
From revistanefrologia.com
Unmeasurable severe hypernatremia A different way of using the How To Calculate Theoretical Osmolality Convert that number to osmoles per liter and add the osmolarity of each. The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. You multiply the molarity by the number of osmoles that each solute produces. Osmolarity cannot be measured but is calculated theoretically from the experimentally measured value of osmolality.. How To Calculate Theoretical Osmolality.
From www.semanticscholar.org
Table 1 from Comparison of methods for calculating serum osmolality How To Calculate Theoretical Osmolality You multiply the molarity by the number of osmoles that each solute produces. To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. Osmolarity cannot be measured but is calculated theoretically from the experimentally measured value of osmolality. The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are. How To Calculate Theoretical Osmolality.
From www.slideserve.com
PPT Diffusion PowerPoint Presentation, free download ID6798349 How To Calculate Theoretical Osmolality An osmole (osmol) is 1 mol of particles that. Convert that number to osmoles per liter and add the osmolarity of each. Osmolality is milliosmoles of solutes per one kilogram (or liter) of water of solution (plasma) and is calculated by osmolarity divided to. Osmolarity of a solution is a theoretical quantity expressed in osmoles per l (osmol per l). How To Calculate Theoretical Osmolality.
From www.slideserve.com
PPT Antidiuretic Hormone ADH PowerPoint Presentation, free download How To Calculate Theoretical Osmolality Osmolality is milliosmoles of solutes per one kilogram (or liter) of water of solution (plasma) and is calculated by osmolarity divided to. Convert that number to osmoles per liter and add the osmolarity of each. An osmole (osmol) is 1 mol of particles that. To calculate the osmolarity of a solution, the first step is to convert the number to. How To Calculate Theoretical Osmolality.
From www.slideserve.com
PPT Osmolar Gaps How does EtOH contribute to osmolar gaps? Can How To Calculate Theoretical Osmolality An osmole (osmol) is 1 mol of particles that. Osmolarity cannot be measured but is calculated theoretically from the experimentally measured value of osmolality. You multiply the molarity by the number of osmoles that each solute produces. Osmolarity of a solution is a theoretical quantity expressed in osmoles per l (osmol per l) of a solution and is widely used. How To Calculate Theoretical Osmolality.
From ar.inspiredpencil.com
Osmolality How To Calculate Theoretical Osmolality You multiply the molarity by the number of osmoles that each solute produces. The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. Convert that number to osmoles per liter and add the osmolarity of each. Osmolarity cannot be measured but is calculated theoretically from the experimentally measured value of osmolality.. How To Calculate Theoretical Osmolality.
From www.coursehero.com
[Solved] I need help figuring out how to calculate molality and How To Calculate Theoretical Osmolality Osmolality is milliosmoles of solutes per one kilogram (or liter) of water of solution (plasma) and is calculated by osmolarity divided to. You multiply the molarity by the number of osmoles that each solute produces. An osmole (osmol) is 1 mol of particles that. The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five. How To Calculate Theoretical Osmolality.
From www.slideserve.com
PPT Chemical calculations used in medicine part 1 PowerPoint How To Calculate Theoretical Osmolality The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. Osmolarity cannot be measured but is calculated theoretically from the experimentally measured value of osmolality. To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. Osmolarity of a solution is a theoretical. How To Calculate Theoretical Osmolality.
From www.toolsbyafiya.com
Osmolar Gap Calculator Tools By Afiya How To Calculate Theoretical Osmolality Osmolarity of a solution is a theoretical quantity expressed in osmoles per l (osmol per l) of a solution and is widely used in clinical practice. Convert that number to osmoles per liter and add the osmolarity of each. The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. To calculate. How To Calculate Theoretical Osmolality.
From present5.com
Diagnosis and Management of Hyperglycemic Crises Diabetic Ketoacidosis How To Calculate Theoretical Osmolality An osmole (osmol) is 1 mol of particles that. Osmolarity cannot be measured but is calculated theoretically from the experimentally measured value of osmolality. Osmolality is milliosmoles of solutes per one kilogram (or liter) of water of solution (plasma) and is calculated by osmolarity divided to. Osmolarity of a solution is a theoretical quantity expressed in osmoles per l (osmol. How To Calculate Theoretical Osmolality.
From www.coursehero.com
[Solved] I need help figuring out how to calculate molality and How To Calculate Theoretical Osmolality Osmolarity of a solution is a theoretical quantity expressed in osmoles per l (osmol per l) of a solution and is widely used in clinical practice. To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. Convert that number to osmoles per liter and add the osmolarity of each. An osmole. How To Calculate Theoretical Osmolality.
From www.youtube.com
Estimating Serum Osmolality using a Simple Formula YouTube How To Calculate Theoretical Osmolality Osmolarity of a solution is a theoretical quantity expressed in osmoles per l (osmol per l) of a solution and is widely used in clinical practice. Osmolality is milliosmoles of solutes per one kilogram (or liter) of water of solution (plasma) and is calculated by osmolarity divided to. You multiply the molarity by the number of osmoles that each solute. How To Calculate Theoretical Osmolality.
From ibiologia.com
Osmolarity Definition, Formula & Osmolarity vs. Osmolality How To Calculate Theoretical Osmolality The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. Osmolarity of a solution is a theoretical quantity expressed in osmoles per l (osmol per l) of a solution and is widely used in clinical practice. Convert that number to osmoles per liter and add the osmolarity of each. You multiply. How To Calculate Theoretical Osmolality.
From www.pinterest.com
Serum osmolality Diffusion / Osmosis Nursing study, Physiology, Rn How To Calculate Theoretical Osmolality The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. Osmolarity cannot be measured but is calculated theoretically from the experimentally measured value of osmolality. Osmolality is milliosmoles of solutes per one kilogram (or liter) of water of solution (plasma) and is calculated by osmolarity divided to. Convert that number to. How To Calculate Theoretical Osmolality.
From www.slideshare.net
Formula osmolality and nutritional needs How To Calculate Theoretical Osmolality The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. You multiply the molarity by the number of osmoles that each solute produces. Osmolality is milliosmoles of solutes per one kilogram (or liter) of water of solution (plasma) and is calculated by osmolarity divided to. Convert that number to osmoles per. How To Calculate Theoretical Osmolality.
From www.youtube.com
Osmolarity Example (Bio) YouTube How To Calculate Theoretical Osmolality Osmolarity of a solution is a theoretical quantity expressed in osmoles per l (osmol per l) of a solution and is widely used in clinical practice. The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. Osmolality is milliosmoles of solutes per one kilogram (or liter) of water of solution (plasma). How To Calculate Theoretical Osmolality.
From ojklmexhbr.blogspot.com
How To Calculate Serum Osmolality In this study, we compared the How To Calculate Theoretical Osmolality Osmolarity of a solution is a theoretical quantity expressed in osmoles per l (osmol per l) of a solution and is widely used in clinical practice. Osmolarity cannot be measured but is calculated theoretically from the experimentally measured value of osmolality. Convert that number to osmoles per liter and add the osmolarity of each. An osmole (osmol) is 1 mol. How To Calculate Theoretical Osmolality.
From exonhkusy.blob.core.windows.net
How To Calculate Osmolality Of Glucose at Robert Villegas blog How To Calculate Theoretical Osmolality Convert that number to osmoles per liter and add the osmolarity of each. To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. Osmolality is milliosmoles of solutes per one kilogram (or liter) of water of solution (plasma) and is calculated by osmolarity divided to. An osmole (osmol) is 1 mol. How To Calculate Theoretical Osmolality.
From www.youtube.com
Calculate Molecular Weight from Osmotic Pressure for Nonideal Solution How To Calculate Theoretical Osmolality You multiply the molarity by the number of osmoles that each solute produces. To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. Osmolality is milliosmoles of solutes per one kilogram (or liter) of water of solution (plasma) and is calculated by osmolarity divided to. Osmolarity of a solution is a. How To Calculate Theoretical Osmolality.
From www.youtube.com
Osmolality vs Osmolarity (with a mnemonic) Physiology and Chemistry How To Calculate Theoretical Osmolality To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. Osmolarity of a solution is a theoretical quantity expressed in osmoles per l (osmol per l) of a solution and is widely. How To Calculate Theoretical Osmolality.
From www.youtube.com
Calculated osmolality and osmolality gap YouTube How To Calculate Theoretical Osmolality Osmolarity of a solution is a theoretical quantity expressed in osmoles per l (osmol per l) of a solution and is widely used in clinical practice. The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. Osmolality is milliosmoles of solutes per one kilogram (or liter) of water of solution (plasma). How To Calculate Theoretical Osmolality.
From www.youtube.com
Chemistry Basics Osmolarity, Osmolality and Tonicity YouTube How To Calculate Theoretical Osmolality Osmolality is milliosmoles of solutes per one kilogram (or liter) of water of solution (plasma) and is calculated by osmolarity divided to. Convert that number to osmoles per liter and add the osmolarity of each. To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. Osmolarity of a solution is a. How To Calculate Theoretical Osmolality.
From sukhjithafsa.blogspot.com
25+ Serum Osmolality Calculator SukhjitHafsa How To Calculate Theoretical Osmolality The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. An osmole (osmol) is 1 mol of particles that. Osmolality is milliosmoles of solutes per one kilogram (or liter) of water of solution (plasma) and is calculated by osmolarity divided to. You multiply the molarity by the number of osmoles that. How To Calculate Theoretical Osmolality.
From www.pinterest.com
Difference Between Osmolarity and Osmolality Definition, Explanation How To Calculate Theoretical Osmolality An osmole (osmol) is 1 mol of particles that. Convert that number to osmoles per liter and add the osmolarity of each. You multiply the molarity by the number of osmoles that each solute produces. Osmolarity cannot be measured but is calculated theoretically from the experimentally measured value of osmolality. To calculate the osmolarity of a solution, the first step. How To Calculate Theoretical Osmolality.
From slideplayer.com
Osmolar Gaps How does EtOH contribute to osmolar gaps? Can osmolar How To Calculate Theoretical Osmolality To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. You multiply the molarity by the number of osmoles that each solute produces. The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. Osmolality is milliosmoles of solutes per one kilogram (or. How To Calculate Theoretical Osmolality.
From www.youtube.com
How to solve osmolarity calculation problems 2 YouTube How To Calculate Theoretical Osmolality Osmolality is milliosmoles of solutes per one kilogram (or liter) of water of solution (plasma) and is calculated by osmolarity divided to. You multiply the molarity by the number of osmoles that each solute produces. An osmole (osmol) is 1 mol of particles that. Osmolarity cannot be measured but is calculated theoretically from the experimentally measured value of osmolality. Convert. How To Calculate Theoretical Osmolality.
From www.thetechedvocate.org
How to calculate osmolality The Tech Edvocate How To Calculate Theoretical Osmolality The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. Osmolality is milliosmoles of solutes per one kilogram (or liter) of water of solution (plasma) and is calculated by osmolarity divided to. To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter.. How To Calculate Theoretical Osmolality.
From www.chegg.com
Solved Problems Calculate the osmolality of the following How To Calculate Theoretical Osmolality Osmolarity cannot be measured but is calculated theoretically from the experimentally measured value of osmolality. Osmolality is milliosmoles of solutes per one kilogram (or liter) of water of solution (plasma) and is calculated by osmolarity divided to. To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. The salts of sodium. How To Calculate Theoretical Osmolality.
From ojklmexhbr.blogspot.com
How To Calculate Serum Osmolality In this study, we compared the How To Calculate Theoretical Osmolality Convert that number to osmoles per liter and add the osmolarity of each. Osmolarity of a solution is a theoretical quantity expressed in osmoles per l (osmol per l) of a solution and is widely used in clinical practice. An osmole (osmol) is 1 mol of particles that. Osmolality is milliosmoles of solutes per one kilogram (or liter) of water. How To Calculate Theoretical Osmolality.
From www.youtube.com
Calculated Osmolality YouTube How To Calculate Theoretical Osmolality An osmole (osmol) is 1 mol of particles that. Osmolality is milliosmoles of solutes per one kilogram (or liter) of water of solution (plasma) and is calculated by osmolarity divided to. To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. Osmolarity of a solution is a theoretical quantity expressed in. How To Calculate Theoretical Osmolality.
From www.youtube.com
How to solve osmolarity calculation problems 3 YouTube How To Calculate Theoretical Osmolality An osmole (osmol) is 1 mol of particles that. Osmolarity of a solution is a theoretical quantity expressed in osmoles per l (osmol per l) of a solution and is widely used in clinical practice. You multiply the molarity by the number of osmoles that each solute produces. Osmolarity cannot be measured but is calculated theoretically from the experimentally measured. How To Calculate Theoretical Osmolality.
From www.slideserve.com
PPT Toxicology Anion Gap, Osmolar Gap & Toxic Alcohols PowerPoint How To Calculate Theoretical Osmolality Convert that number to osmoles per liter and add the osmolarity of each. Osmolarity cannot be measured but is calculated theoretically from the experimentally measured value of osmolality. An osmole (osmol) is 1 mol of particles that. Osmolarity of a solution is a theoretical quantity expressed in osmoles per l (osmol per l) of a solution and is widely used. How To Calculate Theoretical Osmolality.