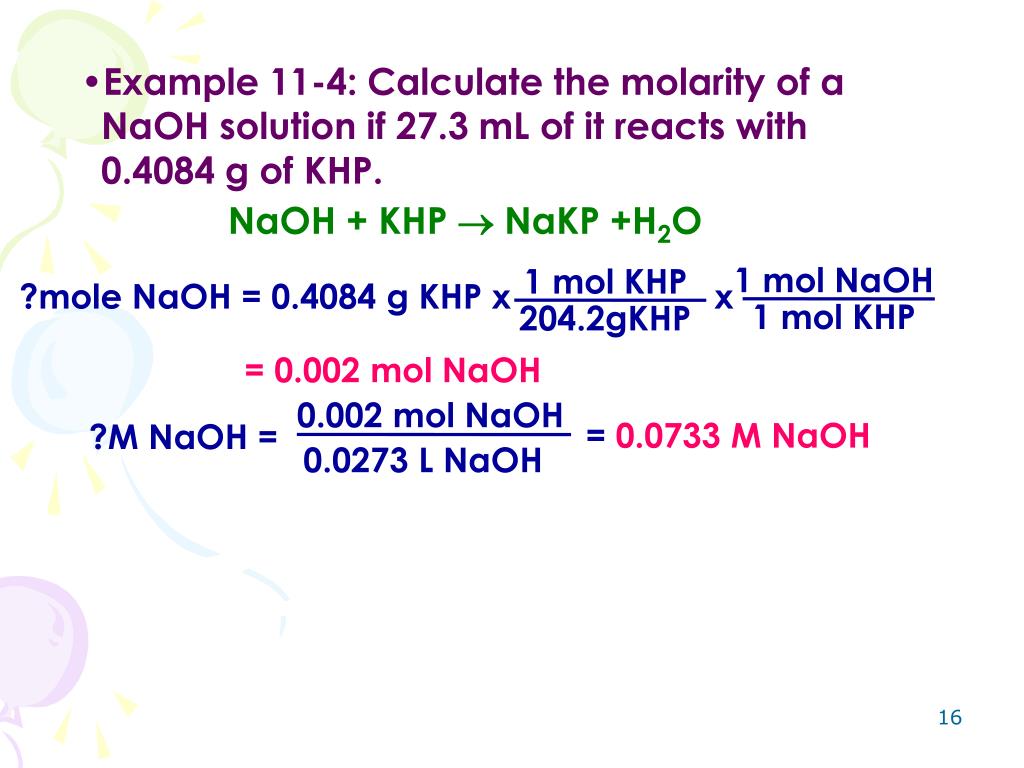
Titration Naoh And Khp . A commonly used primary standard for titration with sodium hydroxide solution is the weak acid potassium hydrogen phthalate or khp (\(\ce{c8h5o4k}\)). In this experiment you will determine the amount of acid present by titration with the strong base naoh. Use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via titration with 25.00. To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using. In this experiment you will use the reaction in equation 3 to determine the molar concentration of the strong base naoh using the weak, monoprotic acid potassium hydrogen. Standardization of a naoh solution using khp an aqueous solution of sodium hydroxide (naoh) was standardized by titrating. You will determine the concentration (standardize) of. Standardization of a naoh solution with potassium hydrogen phthalate (khp) and titration of vinegar with standardized naoh. The reaction between khp and naoh is given below: Since it is hard to prepare a naoh solution of accurately known.
from www.slideserve.com
A commonly used primary standard for titration with sodium hydroxide solution is the weak acid potassium hydrogen phthalate or khp (\(\ce{c8h5o4k}\)). Standardization of a naoh solution with potassium hydrogen phthalate (khp) and titration of vinegar with standardized naoh. In this experiment you will determine the amount of acid present by titration with the strong base naoh. To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using. Use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via titration with 25.00. Since it is hard to prepare a naoh solution of accurately known. Standardization of a naoh solution using khp an aqueous solution of sodium hydroxide (naoh) was standardized by titrating. In this experiment you will use the reaction in equation 3 to determine the molar concentration of the strong base naoh using the weak, monoprotic acid potassium hydrogen. You will determine the concentration (standardize) of. The reaction between khp and naoh is given below:
PPT Reactions in Aqueous Solutions II Calculations PowerPoint
Titration Naoh And Khp In this experiment you will use the reaction in equation 3 to determine the molar concentration of the strong base naoh using the weak, monoprotic acid potassium hydrogen. The reaction between khp and naoh is given below: A commonly used primary standard for titration with sodium hydroxide solution is the weak acid potassium hydrogen phthalate or khp (\(\ce{c8h5o4k}\)). In this experiment you will determine the amount of acid present by titration with the strong base naoh. In this experiment you will use the reaction in equation 3 to determine the molar concentration of the strong base naoh using the weak, monoprotic acid potassium hydrogen. To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using. Standardization of a naoh solution using khp an aqueous solution of sodium hydroxide (naoh) was standardized by titrating. You will determine the concentration (standardize) of. Standardization of a naoh solution with potassium hydrogen phthalate (khp) and titration of vinegar with standardized naoh. Since it is hard to prepare a naoh solution of accurately known. Use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via titration with 25.00.
From www.youtube.com
Standardization of NaOH by titration using KHP YouTube Titration Naoh And Khp A commonly used primary standard for titration with sodium hydroxide solution is the weak acid potassium hydrogen phthalate or khp (\(\ce{c8h5o4k}\)). Use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via titration with 25.00. Standardization of a naoh solution with potassium hydrogen phthalate (khp) and titration of vinegar with standardized naoh. The reaction. Titration Naoh And Khp.
From www.chegg.com
Solved Titration of NaOH with KHP primary standard Molar Titration Naoh And Khp The reaction between khp and naoh is given below: In this experiment you will use the reaction in equation 3 to determine the molar concentration of the strong base naoh using the weak, monoprotic acid potassium hydrogen. Standardization of a naoh solution using khp an aqueous solution of sodium hydroxide (naoh) was standardized by titrating. Standardization of a naoh solution. Titration Naoh And Khp.
From solvedlib.com
Preparing standard KHP and titration with NaOHMolar … SolvedLib Titration Naoh And Khp Since it is hard to prepare a naoh solution of accurately known. Standardization of a naoh solution with potassium hydrogen phthalate (khp) and titration of vinegar with standardized naoh. Standardization of a naoh solution using khp an aqueous solution of sodium hydroxide (naoh) was standardized by titrating. The reaction between khp and naoh is given below: In this experiment you. Titration Naoh And Khp.
From www.chegg.com
Solved Titration of KHP to Determine Concentration of NaOH Titration Naoh And Khp In this experiment you will use the reaction in equation 3 to determine the molar concentration of the strong base naoh using the weak, monoprotic acid potassium hydrogen. Use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via titration with 25.00. You will determine the concentration (standardize) of. A commonly used primary standard. Titration Naoh And Khp.
From www.chegg.com
Solved For each titration of KHP with NaOH solution, Titration Naoh And Khp A commonly used primary standard for titration with sodium hydroxide solution is the weak acid potassium hydrogen phthalate or khp (\(\ce{c8h5o4k}\)). Standardization of a naoh solution with potassium hydrogen phthalate (khp) and titration of vinegar with standardized naoh. You will determine the concentration (standardize) of. Standardization of a naoh solution using khp an aqueous solution of sodium hydroxide (naoh) was. Titration Naoh And Khp.
From www.chegg.com
Solved Titration of KHP to Determine Concentration of NaOH Titration Naoh And Khp Since it is hard to prepare a naoh solution of accurately known. In this experiment you will determine the amount of acid present by titration with the strong base naoh. To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using. Use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to. Titration Naoh And Khp.
From www.youtube.com
Mass of KHP to Standardize a NaOH Solution YouTube Titration Naoh And Khp A commonly used primary standard for titration with sodium hydroxide solution is the weak acid potassium hydrogen phthalate or khp (\(\ce{c8h5o4k}\)). Since it is hard to prepare a naoh solution of accurately known. In this experiment you will use the reaction in equation 3 to determine the molar concentration of the strong base naoh using the weak, monoprotic acid potassium. Titration Naoh And Khp.
From plot.ly
Titration Curve Trial 1 KHP/NaOH line chart made by Alliebally2005 Titration Naoh And Khp You will determine the concentration (standardize) of. Standardization of a naoh solution using khp an aqueous solution of sodium hydroxide (naoh) was standardized by titrating. Standardization of a naoh solution with potassium hydrogen phthalate (khp) and titration of vinegar with standardized naoh. The reaction between khp and naoh is given below: In this experiment you will use the reaction in. Titration Naoh And Khp.
From www.reddit.com
NaOH and KHP titration r/titration Titration Naoh And Khp To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using. A commonly used primary standard for titration with sodium hydroxide solution is the weak acid potassium hydrogen phthalate or khp (\(\ce{c8h5o4k}\)). Since it is hard to prepare a naoh solution of accurately known. In this experiment you will use the reaction in equation. Titration Naoh And Khp.
From www.numerade.com
SOLVED A student carried out two titrations using a NaOH solution of Titration Naoh And Khp Since it is hard to prepare a naoh solution of accurately known. Standardization of a naoh solution with potassium hydrogen phthalate (khp) and titration of vinegar with standardized naoh. To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using. In this experiment you will use the reaction in equation 3 to determine the. Titration Naoh And Khp.
From plotly.com
KHP and NaOH Titration Curve line chart made by Kylclk plotly Titration Naoh And Khp To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using. Standardization of a naoh solution using khp an aqueous solution of sodium hydroxide (naoh) was standardized by titrating. Use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via titration with 25.00. A commonly used primary standard. Titration Naoh And Khp.
From www.scribd.com
CHEM 108 Titration NaOH With KHP F23 PDF Titration Chemistry Titration Naoh And Khp Standardization of a naoh solution using khp an aqueous solution of sodium hydroxide (naoh) was standardized by titrating. Since it is hard to prepare a naoh solution of accurately known. The reaction between khp and naoh is given below: Use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via titration with 25.00. In. Titration Naoh And Khp.
From shotprofessional22.gitlab.io
Ace Khp And Naoh Balanced Equation Physics Wallah 12th Class Chemistry Pdf Titration Naoh And Khp Since it is hard to prepare a naoh solution of accurately known. Standardization of a naoh solution using khp an aqueous solution of sodium hydroxide (naoh) was standardized by titrating. A commonly used primary standard for titration with sodium hydroxide solution is the weak acid potassium hydrogen phthalate or khp (\(\ce{c8h5o4k}\)). In this experiment you will determine the amount of. Titration Naoh And Khp.
From www.youtube.com
Prelab for NaOH Standardization lab YouTube Titration Naoh And Khp Standardization of a naoh solution using khp an aqueous solution of sodium hydroxide (naoh) was standardized by titrating. A commonly used primary standard for titration with sodium hydroxide solution is the weak acid potassium hydrogen phthalate or khp (\(\ce{c8h5o4k}\)). Since it is hard to prepare a naoh solution of accurately known. You will determine the concentration (standardize) of. In this. Titration Naoh And Khp.
From www.numerade.com
SOLVED Question 3 What is the KHP NaOH mole ratio for the reaction Titration Naoh And Khp In this experiment you will determine the amount of acid present by titration with the strong base naoh. To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using. Standardization of a naoh solution using khp an aqueous solution of sodium hydroxide (naoh) was standardized by titrating. The reaction between khp and naoh is. Titration Naoh And Khp.
From www.numerade.com
SOLVEDChemical Analysis by Acid Base Titration Name Part A Titration Naoh And Khp Use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via titration with 25.00. The reaction between khp and naoh is given below: To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using. Standardization of a naoh solution with potassium hydrogen phthalate (khp) and titration of vinegar. Titration Naoh And Khp.
From www.youtube.com
Titration of a monoprotic strong acid (HCl) and monoprotic strong base Titration Naoh And Khp To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using. In this experiment you will use the reaction in equation 3 to determine the molar concentration of the strong base naoh using the weak, monoprotic acid potassium hydrogen. Use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant. Titration Naoh And Khp.
From asideload7.gitlab.io
Nice Khp Naoh Titration Calculations List Of All Physics Formulas Titration Naoh And Khp In this experiment you will determine the amount of acid present by titration with the strong base naoh. You will determine the concentration (standardize) of. Since it is hard to prepare a naoh solution of accurately known. Standardization of a naoh solution using khp an aqueous solution of sodium hydroxide (naoh) was standardized by titrating. The reaction between khp and. Titration Naoh And Khp.
From www.numerade.com
SOLVED Figure 1. KHP and NaOH Titration Curve of pH vs Volume of NaOH Titration Naoh And Khp You will determine the concentration (standardize) of. Standardization of a naoh solution with potassium hydrogen phthalate (khp) and titration of vinegar with standardized naoh. Standardization of a naoh solution using khp an aqueous solution of sodium hydroxide (naoh) was standardized by titrating. In this experiment you will determine the amount of acid present by titration with the strong base naoh.. Titration Naoh And Khp.
From www.slideserve.com
PPT Reactions in Aqueous Solutions II Calculations PowerPoint Titration Naoh And Khp In this experiment you will determine the amount of acid present by titration with the strong base naoh. The reaction between khp and naoh is given below: Since it is hard to prepare a naoh solution of accurately known. Use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via titration with 25.00. A. Titration Naoh And Khp.
From armsingle10.pythonanywhere.com
Breathtaking Titration Of Khp With Naoh Calculations Balancing Titration Naoh And Khp Since it is hard to prepare a naoh solution of accurately known. Standardization of a naoh solution using khp an aqueous solution of sodium hydroxide (naoh) was standardized by titrating. To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using. You will determine the concentration (standardize) of. In this experiment you will use. Titration Naoh And Khp.
From www.chegg.com
draw a titration curve of NaOH and KHP draw a Titration Naoh And Khp You will determine the concentration (standardize) of. Since it is hard to prepare a naoh solution of accurately known. To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using. Standardization of a naoh solution using khp an aqueous solution of sodium hydroxide (naoh) was standardized by titrating. A commonly used primary standard for. Titration Naoh And Khp.
From www.chegg.com
Solved KHP Titration Report Sheet Concentration of NaOH Titration Naoh And Khp To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using. Standardization of a naoh solution using khp an aqueous solution of sodium hydroxide (naoh) was standardized by titrating. Use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via titration with 25.00. In this experiment you will. Titration Naoh And Khp.
From www.coursehero.com
[Solved] Chem. This lab exercise is a titration experiment between KHP Titration Naoh And Khp The reaction between khp and naoh is given below: In this experiment you will use the reaction in equation 3 to determine the molar concentration of the strong base naoh using the weak, monoprotic acid potassium hydrogen. In this experiment you will determine the amount of acid present by titration with the strong base naoh. Standardization of a naoh solution. Titration Naoh And Khp.
From plotly.com
First Derivative of KHP and NaOH Titration Curve line chart made by Titration Naoh And Khp Use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via titration with 25.00. A commonly used primary standard for titration with sodium hydroxide solution is the weak acid potassium hydrogen phthalate or khp (\(\ce{c8h5o4k}\)). In this experiment you will use the reaction in equation 3 to determine the molar concentration of the strong. Titration Naoh And Khp.
From www.numerade.com
SOLVED Part III Titration Standardization of NaOH solution (finding Titration Naoh And Khp Standardization of a naoh solution using khp an aqueous solution of sodium hydroxide (naoh) was standardized by titrating. A commonly used primary standard for titration with sodium hydroxide solution is the weak acid potassium hydrogen phthalate or khp (\(\ce{c8h5o4k}\)). To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using. Standardization of a naoh. Titration Naoh And Khp.
From armsingle10.pythonanywhere.com
Breathtaking Titration Of Khp With Naoh Calculations Balancing Titration Naoh And Khp You will determine the concentration (standardize) of. The reaction between khp and naoh is given below: Use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via titration with 25.00. To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using. In this experiment you will use the. Titration Naoh And Khp.
From www.numerade.com
SOLVEDFor titration of a NaOH solution, HKC8H4O4 (KHP) is used as the Titration Naoh And Khp The reaction between khp and naoh is given below: You will determine the concentration (standardize) of. A commonly used primary standard for titration with sodium hydroxide solution is the weak acid potassium hydrogen phthalate or khp (\(\ce{c8h5o4k}\)). Since it is hard to prepare a naoh solution of accurately known. Standardization of a naoh solution with potassium hydrogen phthalate (khp) and. Titration Naoh And Khp.
From studylib.net
CHM 115 Lab 3 Titration Standardize NaOH/Determine impure KHP Titration Naoh And Khp Use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via titration with 25.00. You will determine the concentration (standardize) of. In this experiment you will use the reaction in equation 3 to determine the molar concentration of the strong base naoh using the weak, monoprotic acid potassium hydrogen. A commonly used primary standard. Titration Naoh And Khp.
From www.numerade.com
SOLVED The titration graph below is the titration of the solution of Titration Naoh And Khp Standardization of a naoh solution with potassium hydrogen phthalate (khp) and titration of vinegar with standardized naoh. A commonly used primary standard for titration with sodium hydroxide solution is the weak acid potassium hydrogen phthalate or khp (\(\ce{c8h5o4k}\)). To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using. You will determine the concentration. Titration Naoh And Khp.
From asideload7.gitlab.io
Nice Khp Naoh Titration Calculations List Of All Physics Formulas Titration Naoh And Khp In this experiment you will use the reaction in equation 3 to determine the molar concentration of the strong base naoh using the weak, monoprotic acid potassium hydrogen. A commonly used primary standard for titration with sodium hydroxide solution is the weak acid potassium hydrogen phthalate or khp (\(\ce{c8h5o4k}\)). Standardization of a naoh solution with potassium hydrogen phthalate (khp) and. Titration Naoh And Khp.
From www.vrogue.co
Standardization Of Naoh With A Khp Solution Acid Base vrogue.co Titration Naoh And Khp To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using. In this experiment you will determine the amount of acid present by titration with the strong base naoh. A commonly used primary standard for titration with sodium hydroxide solution is the weak acid potassium hydrogen phthalate or khp (\(\ce{c8h5o4k}\)). You will determine the. Titration Naoh And Khp.
From armsingle10.pythonanywhere.com
Breathtaking Titration Of Khp With Naoh Calculations Balancing Titration Naoh And Khp To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using. Since it is hard to prepare a naoh solution of accurately known. In this experiment you will use the reaction in equation 3 to determine the molar concentration of the strong base naoh using the weak, monoprotic acid potassium hydrogen. A commonly used. Titration Naoh And Khp.
From www.numerade.com
SOLVEDBonus Question NaOH was standardized by titration against a Titration Naoh And Khp Standardization of a naoh solution with potassium hydrogen phthalate (khp) and titration of vinegar with standardized naoh. To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using. You will determine the concentration (standardize) of. The reaction between khp and naoh is given below: Since it is hard to prepare a naoh solution of. Titration Naoh And Khp.
From www.numerade.com
SOLVED Question 3 Please Experiment 5 AcidBase Titration Titration Naoh And Khp In this experiment you will determine the amount of acid present by titration with the strong base naoh. In this experiment you will use the reaction in equation 3 to determine the molar concentration of the strong base naoh using the weak, monoprotic acid potassium hydrogen. Since it is hard to prepare a naoh solution of accurately known. Standardization of. Titration Naoh And Khp.