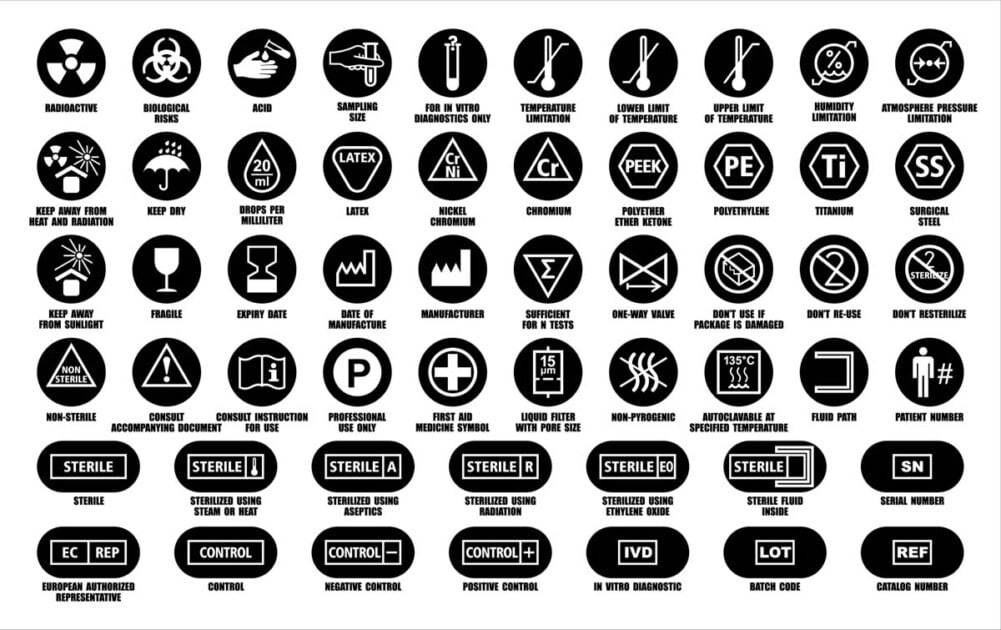
Labeling Medical Device Packaging . Specify the general content and format of medical device and ivd medical device labelling in paper or electronic format. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical device submissions. Design includes labeling content that. Labelling serves to communicate safety and performance related information to users of medical devices and/or. Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Iso 13485:2016 requires that “labeling” be part of the medical device file. During production, monitoring that labeling and packaging are compliant is necessary.
from exyrvtbcz.blob.core.windows.net
Specify the general content and format of medical device and ivd medical device labelling in paper or electronic format. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical device submissions. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. During production, monitoring that labeling and packaging are compliant is necessary. Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. Iso 13485:2016 requires that “labeling” be part of the medical device file. Design includes labeling content that. Labelling serves to communicate safety and performance related information to users of medical devices and/or.
Symbols In Medical Device Labeling at Jillian Bundy blog
Labeling Medical Device Packaging Iso 13485:2016 requires that “labeling” be part of the medical device file. During production, monitoring that labeling and packaging are compliant is necessary. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical device submissions. Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. Labelling serves to communicate safety and performance related information to users of medical devices and/or. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Specify the general content and format of medical device and ivd medical device labelling in paper or electronic format. Iso 13485:2016 requires that “labeling” be part of the medical device file. Design includes labeling content that.
From www.ossid.com
Advanced Medical Device & Pharmacuetical Packaging Solutions Ossid Labeling Medical Device Packaging Specify the general content and format of medical device and ivd medical device labelling in paper or electronic format. Design includes labeling content that. Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. Adequate labeling for a medical device requires proper design and procurement of the labels. Labeling Medical Device Packaging.
From www.docdroid.net
Medical Device packaging & Labeling Symbols.pdf DocDroid Labeling Medical Device Packaging Design includes labeling content that. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical device submissions. Labelling serves to communicate safety and performance related information to users of medical devices and/or. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Iso 13485:2016 requires that “labeling”. Labeling Medical Device Packaging.
From clin-r.com
Labels for Medical Devices Clin R Labeling Medical Device Packaging Specify the general content and format of medical device and ivd medical device labelling in paper or electronic format. During production, monitoring that labeling and packaging are compliant is necessary. Design includes labeling content that. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical device submissions. Iso 13485:2016 requires that “labeling” be. Labeling Medical Device Packaging.
From www.gmdesigndevelopment.com
MEDICAL DEVICE SYMBOLS Gm Design Development UK Labeling Medical Device Packaging Labelling serves to communicate safety and performance related information to users of medical devices and/or. During production, monitoring that labeling and packaging are compliant is necessary. Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. Design includes labeling content that. •understand the requirements for medical device labeling. Labeling Medical Device Packaging.
From vascufirst.com
What is the meaning of symbols on medical devices labels? VascuFirst Labeling Medical Device Packaging Design includes labeling content that. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Iso 13485:2016 requires that “labeling” be part of the medical device file. Labelling serves to communicate safety and performance related information to users of medical devices and/or. •understand the requirements for medical device labeling •review some key labeling provisions. Labeling Medical Device Packaging.
From loeakndnh.blob.core.windows.net
Label The Device at Dale Sage blog Labeling Medical Device Packaging Specify the general content and format of medical device and ivd medical device labelling in paper or electronic format. Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Design includes labeling. Labeling Medical Device Packaging.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Labeling Medical Device Packaging Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Specify the general content and format of medical device and ivd medical device labelling in paper or electronic format. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical device submissions. Iso 13485:2016 requires that “labeling” be. Labeling Medical Device Packaging.
From exyrvtbcz.blob.core.windows.net
Symbols In Medical Device Labeling at Jillian Bundy blog Labeling Medical Device Packaging Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. During production, monitoring that labeling and packaging are compliant is necessary. Labelling serves to communicate safety and performance related information to users of medical devices and/or. •understand the requirements for medical device labeling •review some key labeling provisions. Labeling Medical Device Packaging.
From vanderstahl.com
Critical Importance of Medical device Packaging Testing Van der Stahl Labeling Medical Device Packaging Design includes labeling content that. During production, monitoring that labeling and packaging are compliant is necessary. Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. •understand the requirements for medical device. Labeling Medical Device Packaging.
From easymedicaldevice.com
How to Create a Label as per EU MDR 2017/745? Labeling Medical Device Packaging Design includes labeling content that. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical device submissions. Specify the general content and format of medical device and ivd medical device labelling in paper or electronic format. Iso 13485:2016 requires that “labeling” be part of the medical device file. During production, monitoring that labeling. Labeling Medical Device Packaging.
From mungfali.com
Medical Device Labeling Symbols Labeling Medical Device Packaging During production, monitoring that labeling and packaging are compliant is necessary. Specify the general content and format of medical device and ivd medical device labelling in paper or electronic format. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical device submissions. Labelling serves to communicate safety and performance related information to users. Labeling Medical Device Packaging.
From www.alamy.com
Five medical symbols about the method of sterilization on medical Labeling Medical Device Packaging Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Design includes labeling content that. During production, monitoring that labeling and packaging are compliant is necessary. Specify the general content and format of medical device and ivd medical device labelling in paper or electronic format. Labeling and packaging is key to ensure that a. Labeling Medical Device Packaging.
From www.vecteezy.com
Label pack for medicinal tablets, label medicine paper design, medicine Labeling Medical Device Packaging During production, monitoring that labeling and packaging are compliant is necessary. Specify the general content and format of medical device and ivd medical device labelling in paper or electronic format. Design includes labeling content that. Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. Iso 13485:2016 requires. Labeling Medical Device Packaging.
From easymedicaldevice.com
How to Create a Label as per EU MDR 2017/745? Labeling Medical Device Packaging Labelling serves to communicate safety and performance related information to users of medical devices and/or. During production, monitoring that labeling and packaging are compliant is necessary. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical device submissions. Adequate labeling for a medical device requires proper design and procurement of the labels and. Labeling Medical Device Packaging.
From medicaldevicelicense.com
Essential Medical Device Symbols for Labeling ISO 152231 Labeling Medical Device Packaging Iso 13485:2016 requires that “labeling” be part of the medical device file. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical device submissions. Specify the general content and format of medical device and ivd medical device labelling in paper or electronic format. Labeling and packaging is key to ensure that a medical. Labeling Medical Device Packaging.
From www.medicalcarts.org
Packaging Symbols for Your Medical Cart Shipment (Infographic) Labeling Medical Device Packaging Iso 13485:2016 requires that “labeling” be part of the medical device file. Specify the general content and format of medical device and ivd medical device labelling in paper or electronic format. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. •understand the requirements for medical device labeling •review some key labeling provisions for. Labeling Medical Device Packaging.
From klaiqkgel.blob.core.windows.net
Medical Device Labeling Requirements Australia at Peter Rameriz blog Labeling Medical Device Packaging Design includes labeling content that. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Labelling serves to communicate safety and performance related information to users of medical devices and/or. Specify the general content and format of medical device and ivd medical device labelling in paper or electronic format. Iso 13485:2016 requires that “labeling”. Labeling Medical Device Packaging.
From exyzsultp.blob.core.windows.net
Fda Guidance Medical Device Patient Labeling at Jana Flores blog Labeling Medical Device Packaging Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Labelling serves to communicate safety and performance related information to users of medical devices and/or. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical device submissions. Design includes labeling content that. Labeling and packaging is key. Labeling Medical Device Packaging.
From clin-r.com
Labels for Medical Devices Clin R Labeling Medical Device Packaging Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. During production, monitoring that labeling and packaging are compliant is necessary. Iso 13485:2016 requires that “labeling” be part of the medical device file. Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and. Labeling Medical Device Packaging.
From www.kmedhealth.com
Medical Device Label SymbolsI In Packaging Labeling Medical Device Packaging Design includes labeling content that. Labelling serves to communicate safety and performance related information to users of medical devices and/or. During production, monitoring that labeling and packaging are compliant is necessary. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Specify the general content and format of medical device and ivd medical device. Labeling Medical Device Packaging.
From mungfali.com
Medical Device Labeling Symbols Labeling Medical Device Packaging Design includes labeling content that. During production, monitoring that labeling and packaging are compliant is necessary. Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Iso 13485:2016 requires that “labeling” be. Labeling Medical Device Packaging.
From www.alamy.com
Full set of medical device packaging symbols with warning information Labeling Medical Device Packaging Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Specify the general content and format of medical device and ivd medical device labelling in paper or electronic format. Design includes labeling content that. During production, monitoring that labeling and packaging are compliant is necessary. •understand the requirements for medical device labeling •review some. Labeling Medical Device Packaging.
From www.packagingconnections.com
Labelling things to know in Pharmaceutical Drugs & Medical Devices Labeling Medical Device Packaging Labelling serves to communicate safety and performance related information to users of medical devices and/or. Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. Iso 13485:2016 requires that “labeling” be part of the medical device file. Design includes labeling content that. During production, monitoring that labeling and. Labeling Medical Device Packaging.
From easymedicaldevice.com
How to Create a Label as per EU MDR 2017/745? Labeling Medical Device Packaging Specify the general content and format of medical device and ivd medical device labelling in paper or electronic format. Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. Iso 13485:2016 requires that “labeling” be part of the medical device file. Labelling serves to communicate safety and performance. Labeling Medical Device Packaging.
From dandelionsandthings.blogspot.com
30 Medical Device Label Symbols Label Design Ideas 2020 Labeling Medical Device Packaging Design includes labeling content that. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical device submissions. Iso 13485:2016 requires that “labeling” be part of the medical device file. Specify the general content and format of medical. Labeling Medical Device Packaging.
From clin-r.com
Labels for Medical Devices Clin R Labeling Medical Device Packaging Labelling serves to communicate safety and performance related information to users of medical devices and/or. Iso 13485:2016 requires that “labeling” be part of the medical device file. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical device submissions. Specify the general content and format of medical device and ivd medical device labelling. Labeling Medical Device Packaging.
From klaiqkgel.blob.core.windows.net
Medical Device Labeling Requirements Australia at Peter Rameriz blog Labeling Medical Device Packaging Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. Labelling serves to communicate safety and performance related information to users of medical devices and/or. During production, monitoring that labeling and packaging are compliant is necessary. Design includes labeling content that. Iso 13485:2016 requires that “labeling” be part. Labeling Medical Device Packaging.
From packoi.com
How to Design an Effective Packaging Label that Communicates Your Brand Labeling Medical Device Packaging During production, monitoring that labeling and packaging are compliant is necessary. Labelling serves to communicate safety and performance related information to users of medical devices and/or. Specify the general content and format of medical device and ivd medical device labelling in paper or electronic format. Iso 13485:2016 requires that “labeling” be part of the medical device file. Adequate labeling for. Labeling Medical Device Packaging.
From www.regdesk.co
FDA on General Principles of Labeling for Medical Devices RegDesk Labeling Medical Device Packaging Iso 13485:2016 requires that “labeling” be part of the medical device file. Design includes labeling content that. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. Specify the general content and. Labeling Medical Device Packaging.
From c10.beauty
Packaging Symbols And What They Mean Labeling Medical Device Packaging Specify the general content and format of medical device and ivd medical device labelling in paper or electronic format. Labelling serves to communicate safety and performance related information to users of medical devices and/or. Iso 13485:2016 requires that “labeling” be part of the medical device file. Labeling and packaging is key to ensure that a medical device or in vitro. Labeling Medical Device Packaging.
From www.team-consulting.com
Medical device packaging design Team Consulting Labeling Medical Device Packaging Specify the general content and format of medical device and ivd medical device labelling in paper or electronic format. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. During production, monitoring that labeling and packaging are compliant is necessary. Iso 13485:2016 requires that “labeling” be part of the medical device file. Labeling and. Labeling Medical Device Packaging.
From tataelxsi.com
Tata Elxsi Medical Device Packaging & Labeling Services Labeling Medical Device Packaging Specify the general content and format of medical device and ivd medical device labelling in paper or electronic format. •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical device submissions. Design includes labeling content that. Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can. Labeling Medical Device Packaging.
From clin-r.com
Labels for Medical Devices Clin R Labeling Medical Device Packaging Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. During production, monitoring that labeling and packaging are compliant is necessary. Design includes labeling content that. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Labelling serves to communicate safety and. Labeling Medical Device Packaging.
From medicaldevicelicense.com
Essential Medical Device Symbols for Labeling ISO 152231 Labeling Medical Device Packaging •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical device submissions. Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. During production, monitoring that labeling and packaging are compliant is necessary. Labelling serves to communicate safety and performance related. Labeling Medical Device Packaging.
From labelservice.co.uk
Medical Device Labels, Medical Device Labelling Labelservice Labeling Medical Device Packaging •understand the requirements for medical device labeling •review some key labeling provisions for different types of medical device submissions. Iso 13485:2016 requires that “labeling” be part of the medical device file. During production, monitoring that labeling and packaging are compliant is necessary. Specify the general content and format of medical device and ivd medical device labelling in paper or electronic. Labeling Medical Device Packaging.