Zinc Carbonate Equation Thermal Decomposition . A metal carbonate that needs to be. It describes and explains how. The relative ease with which the carbonates of some of the less reactive metals are decomposed has been used in the extraction of these metals from ores that contain the metal as a carbonate, for example zinc carbonate (calamine). In this video, i will explain the thermal decomposition of zinc carbonate/subscribe & hit. Alkaline earth metal carbonates will decompose when heated making the metal oxide and carbon dioxide gas. In a thermal decomposition reaction, there is only one reactant, but two or more products. Zinc carbonate breaks up to form zinc oxide and carbon dioxide. The equation for the reaction is: Thermal decomposition of zinc carbonate. You could see the effect of the gas. Breaking a compound down into. The group 2 carbonates break down (decompose) when they are heated to form the metal oxide and give off. Cuco 3 (s) → cuo (s) + co 2 (g) copper(ii) carbonate → copper(ii) oxide + carbon dioxide.
from www.numerade.com
In a thermal decomposition reaction, there is only one reactant, but two or more products. In this video, i will explain the thermal decomposition of zinc carbonate/subscribe & hit. It describes and explains how. Alkaline earth metal carbonates will decompose when heated making the metal oxide and carbon dioxide gas. Breaking a compound down into. The group 2 carbonates break down (decompose) when they are heated to form the metal oxide and give off. Thermal decomposition of zinc carbonate. The equation for the reaction is: Zinc carbonate breaks up to form zinc oxide and carbon dioxide. Cuco 3 (s) → cuo (s) + co 2 (g) copper(ii) carbonate → copper(ii) oxide + carbon dioxide.
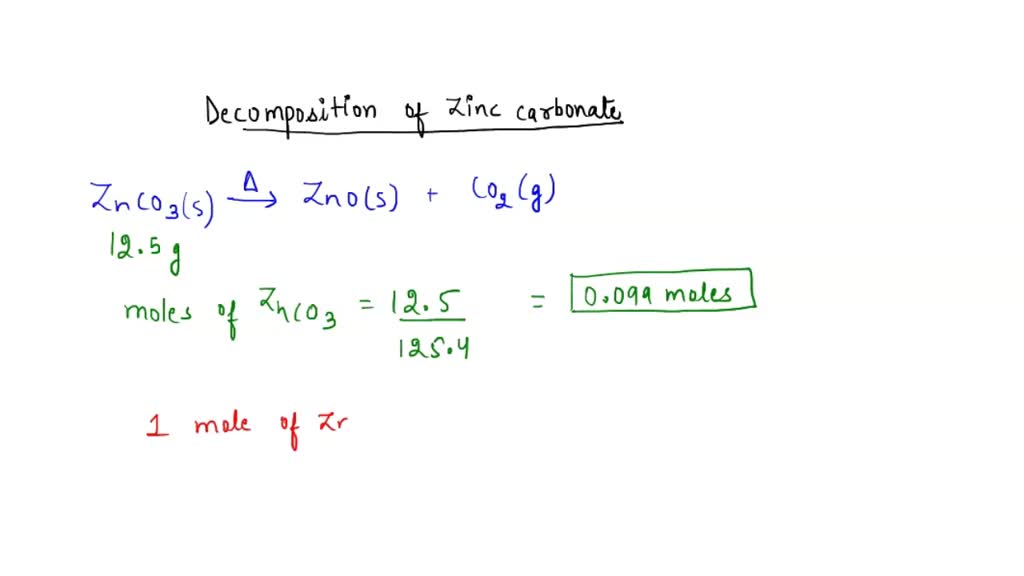
12.5g of zinc carbonate is heated, it to make 8.1g of zinc
Zinc Carbonate Equation Thermal Decomposition Alkaline earth metal carbonates will decompose when heated making the metal oxide and carbon dioxide gas. Zinc carbonate breaks up to form zinc oxide and carbon dioxide. You could see the effect of the gas. In this video, i will explain the thermal decomposition of zinc carbonate/subscribe & hit. In a thermal decomposition reaction, there is only one reactant, but two or more products. The group 2 carbonates break down (decompose) when they are heated to form the metal oxide and give off. Breaking a compound down into. Alkaline earth metal carbonates will decompose when heated making the metal oxide and carbon dioxide gas. It describes and explains how. Thermal decomposition of zinc carbonate. A metal carbonate that needs to be. The equation for the reaction is: The relative ease with which the carbonates of some of the less reactive metals are decomposed has been used in the extraction of these metals from ores that contain the metal as a carbonate, for example zinc carbonate (calamine). Cuco 3 (s) → cuo (s) + co 2 (g) copper(ii) carbonate → copper(ii) oxide + carbon dioxide.
From www.slideshare.net
Action of heat on chemical compound Zinc Carbonate Equation Thermal Decomposition Alkaline earth metal carbonates will decompose when heated making the metal oxide and carbon dioxide gas. The equation for the reaction is: The group 2 carbonates break down (decompose) when they are heated to form the metal oxide and give off. Zinc carbonate breaks up to form zinc oxide and carbon dioxide. You could see the effect of the gas.. Zinc Carbonate Equation Thermal Decomposition.
From www.shutterstock.com
Reaction Infographic Diagram Example Zinc Stock Vector Zinc Carbonate Equation Thermal Decomposition You could see the effect of the gas. Cuco 3 (s) → cuo (s) + co 2 (g) copper(ii) carbonate → copper(ii) oxide + carbon dioxide. Breaking a compound down into. It describes and explains how. Thermal decomposition of zinc carbonate. The equation for the reaction is: Zinc carbonate breaks up to form zinc oxide and carbon dioxide. A metal. Zinc Carbonate Equation Thermal Decomposition.
From www.youtube.com
Thermal of Zinc Carbonate YouTube Zinc Carbonate Equation Thermal Decomposition The equation for the reaction is: Alkaline earth metal carbonates will decompose when heated making the metal oxide and carbon dioxide gas. The relative ease with which the carbonates of some of the less reactive metals are decomposed has been used in the extraction of these metals from ores that contain the metal as a carbonate, for example zinc carbonate. Zinc Carbonate Equation Thermal Decomposition.
From www.researchgate.net
(PDF) The of Zinc Carbonate Using Stoichiometry To Zinc Carbonate Equation Thermal Decomposition The equation for the reaction is: Thermal decomposition of zinc carbonate. The group 2 carbonates break down (decompose) when they are heated to form the metal oxide and give off. You could see the effect of the gas. In this video, i will explain the thermal decomposition of zinc carbonate/subscribe & hit. In a thermal decomposition reaction, there is only. Zinc Carbonate Equation Thermal Decomposition.
From slideplayer.com
Combination 2. Precipitation 3. Thermal ppt download Zinc Carbonate Equation Thermal Decomposition The equation for the reaction is: In a thermal decomposition reaction, there is only one reactant, but two or more products. It describes and explains how. Zinc carbonate breaks up to form zinc oxide and carbon dioxide. Alkaline earth metal carbonates will decompose when heated making the metal oxide and carbon dioxide gas. Breaking a compound down into. In this. Zinc Carbonate Equation Thermal Decomposition.
From www.youtube.com
Type of Reaction for ZnCO3 = ZnO + CO2 YouTube Zinc Carbonate Equation Thermal Decomposition Breaking a compound down into. Thermal decomposition of zinc carbonate. Alkaline earth metal carbonates will decompose when heated making the metal oxide and carbon dioxide gas. A metal carbonate that needs to be. It describes and explains how. Cuco 3 (s) → cuo (s) + co 2 (g) copper(ii) carbonate → copper(ii) oxide + carbon dioxide. Zinc carbonate breaks up. Zinc Carbonate Equation Thermal Decomposition.
From www.youtube.com
Thermal of Zinc Carbonate YouTube Zinc Carbonate Equation Thermal Decomposition The equation for the reaction is: In this video, i will explain the thermal decomposition of zinc carbonate/subscribe & hit. Alkaline earth metal carbonates will decompose when heated making the metal oxide and carbon dioxide gas. It describes and explains how. The relative ease with which the carbonates of some of the less reactive metals are decomposed has been used. Zinc Carbonate Equation Thermal Decomposition.
From www.researchgate.net
(PDF) Influences of evolved gases on the thermal of zinc Zinc Carbonate Equation Thermal Decomposition Breaking a compound down into. It describes and explains how. In a thermal decomposition reaction, there is only one reactant, but two or more products. You could see the effect of the gas. Thermal decomposition of zinc carbonate. In this video, i will explain the thermal decomposition of zinc carbonate/subscribe & hit. The equation for the reaction is: Zinc carbonate. Zinc Carbonate Equation Thermal Decomposition.
From www.numerade.com
The of zinc carbonate, ZnCO3( s), into zinc oxide, ZnO(s Zinc Carbonate Equation Thermal Decomposition In a thermal decomposition reaction, there is only one reactant, but two or more products. It describes and explains how. Breaking a compound down into. You could see the effect of the gas. The relative ease with which the carbonates of some of the less reactive metals are decomposed has been used in the extraction of these metals from ores. Zinc Carbonate Equation Thermal Decomposition.
From www.scielo.br
SciELO Brasil Synthesis and Characterization of Zinc Oxide Obtained Zinc Carbonate Equation Thermal Decomposition A metal carbonate that needs to be. Zinc carbonate breaks up to form zinc oxide and carbon dioxide. Alkaline earth metal carbonates will decompose when heated making the metal oxide and carbon dioxide gas. In a thermal decomposition reaction, there is only one reactant, but two or more products. You could see the effect of the gas. The relative ease. Zinc Carbonate Equation Thermal Decomposition.
From www.youtube.com
How to Write the Formula for ZnCO3 (Zinc carbonate) YouTube Zinc Carbonate Equation Thermal Decomposition In a thermal decomposition reaction, there is only one reactant, but two or more products. It describes and explains how. In this video, i will explain the thermal decomposition of zinc carbonate/subscribe & hit. Breaking a compound down into. Zinc carbonate breaks up to form zinc oxide and carbon dioxide. Cuco 3 (s) → cuo (s) + co 2 (g). Zinc Carbonate Equation Thermal Decomposition.
From www.slideserve.com
PPT What is a chemical reaction? PowerPoint Presentation, free Zinc Carbonate Equation Thermal Decomposition Thermal decomposition of zinc carbonate. The group 2 carbonates break down (decompose) when they are heated to form the metal oxide and give off. The relative ease with which the carbonates of some of the less reactive metals are decomposed has been used in the extraction of these metals from ores that contain the metal as a carbonate, for example. Zinc Carbonate Equation Thermal Decomposition.
From hxeheqnhn.blob.core.windows.net
Zinc Carbonate Equation at John Baer blog Zinc Carbonate Equation Thermal Decomposition Cuco 3 (s) → cuo (s) + co 2 (g) copper(ii) carbonate → copper(ii) oxide + carbon dioxide. The relative ease with which the carbonates of some of the less reactive metals are decomposed has been used in the extraction of these metals from ores that contain the metal as a carbonate, for example zinc carbonate (calamine). The equation for. Zinc Carbonate Equation Thermal Decomposition.
From www.nagwa.com
Question Video Identifying the Correct Chemical Equation for the Zinc Carbonate Equation Thermal Decomposition Breaking a compound down into. Cuco 3 (s) → cuo (s) + co 2 (g) copper(ii) carbonate → copper(ii) oxide + carbon dioxide. It describes and explains how. You could see the effect of the gas. Alkaline earth metal carbonates will decompose when heated making the metal oxide and carbon dioxide gas. The equation for the reaction is: In this. Zinc Carbonate Equation Thermal Decomposition.
From studylib.net
File Zinc Carbonate Equation Thermal Decomposition Cuco 3 (s) → cuo (s) + co 2 (g) copper(ii) carbonate → copper(ii) oxide + carbon dioxide. The relative ease with which the carbonates of some of the less reactive metals are decomposed has been used in the extraction of these metals from ores that contain the metal as a carbonate, for example zinc carbonate (calamine). You could see. Zinc Carbonate Equation Thermal Decomposition.
From www.slideserve.com
PPT Thermal PowerPoint Presentation ID544610 Zinc Carbonate Equation Thermal Decomposition You could see the effect of the gas. Zinc carbonate breaks up to form zinc oxide and carbon dioxide. In this video, i will explain the thermal decomposition of zinc carbonate/subscribe & hit. Alkaline earth metal carbonates will decompose when heated making the metal oxide and carbon dioxide gas. A metal carbonate that needs to be. In a thermal decomposition. Zinc Carbonate Equation Thermal Decomposition.
From www.online-sciences.com
Types of chemical reactions and Thermal reactions Zinc Carbonate Equation Thermal Decomposition Alkaline earth metal carbonates will decompose when heated making the metal oxide and carbon dioxide gas. Breaking a compound down into. In a thermal decomposition reaction, there is only one reactant, but two or more products. You could see the effect of the gas. Zinc carbonate breaks up to form zinc oxide and carbon dioxide. It describes and explains how.. Zinc Carbonate Equation Thermal Decomposition.
From www.savemyexams.com
Carbon Dioxide from Thermal Edexcel IGCSE Chemistry Zinc Carbonate Equation Thermal Decomposition Breaking a compound down into. It describes and explains how. The group 2 carbonates break down (decompose) when they are heated to form the metal oxide and give off. A metal carbonate that needs to be. You could see the effect of the gas. Alkaline earth metal carbonates will decompose when heated making the metal oxide and carbon dioxide gas.. Zinc Carbonate Equation Thermal Decomposition.
From hxeheqnhn.blob.core.windows.net
Zinc Carbonate Equation at John Baer blog Zinc Carbonate Equation Thermal Decomposition Breaking a compound down into. The group 2 carbonates break down (decompose) when they are heated to form the metal oxide and give off. In a thermal decomposition reaction, there is only one reactant, but two or more products. The equation for the reaction is: Cuco 3 (s) → cuo (s) + co 2 (g) copper(ii) carbonate → copper(ii) oxide. Zinc Carbonate Equation Thermal Decomposition.
From www.numerade.com
12.5g of zinc carbonate is heated, it to make 8.1g of zinc Zinc Carbonate Equation Thermal Decomposition Cuco 3 (s) → cuo (s) + co 2 (g) copper(ii) carbonate → copper(ii) oxide + carbon dioxide. In this video, i will explain the thermal decomposition of zinc carbonate/subscribe & hit. The relative ease with which the carbonates of some of the less reactive metals are decomposed has been used in the extraction of these metals from ores that. Zinc Carbonate Equation Thermal Decomposition.
From www.nagwa.com
Question Video Finding the Mass of the Products in a Thermal Zinc Carbonate Equation Thermal Decomposition Alkaline earth metal carbonates will decompose when heated making the metal oxide and carbon dioxide gas. Cuco 3 (s) → cuo (s) + co 2 (g) copper(ii) carbonate → copper(ii) oxide + carbon dioxide. A metal carbonate that needs to be. The relative ease with which the carbonates of some of the less reactive metals are decomposed has been used. Zinc Carbonate Equation Thermal Decomposition.
From www.slideserve.com
PPT The limestone cycle PowerPoint Presentation ID224629 Zinc Carbonate Equation Thermal Decomposition The group 2 carbonates break down (decompose) when they are heated to form the metal oxide and give off. Cuco 3 (s) → cuo (s) + co 2 (g) copper(ii) carbonate → copper(ii) oxide + carbon dioxide. Breaking a compound down into. It describes and explains how. You could see the effect of the gas. In this video, i will. Zinc Carbonate Equation Thermal Decomposition.
From www.researchgate.net
(PDF) Effect of CO2 partial pressure on the thermal Zinc Carbonate Equation Thermal Decomposition A metal carbonate that needs to be. Cuco 3 (s) → cuo (s) + co 2 (g) copper(ii) carbonate → copper(ii) oxide + carbon dioxide. It describes and explains how. In a thermal decomposition reaction, there is only one reactant, but two or more products. You could see the effect of the gas. Thermal decomposition of zinc carbonate. Alkaline earth. Zinc Carbonate Equation Thermal Decomposition.
From www.researchgate.net
Thermal profile of zinc hydroxide nitrate treated in (a Zinc Carbonate Equation Thermal Decomposition You could see the effect of the gas. The group 2 carbonates break down (decompose) when they are heated to form the metal oxide and give off. In this video, i will explain the thermal decomposition of zinc carbonate/subscribe & hit. Thermal decomposition of zinc carbonate. In a thermal decomposition reaction, there is only one reactant, but two or more. Zinc Carbonate Equation Thermal Decomposition.
From www.semanticscholar.org
Figure 1 from Synthesis of Zno Nanoparticles by Thermal Zinc Carbonate Equation Thermal Decomposition The equation for the reaction is: Alkaline earth metal carbonates will decompose when heated making the metal oxide and carbon dioxide gas. Zinc carbonate breaks up to form zinc oxide and carbon dioxide. In this video, i will explain the thermal decomposition of zinc carbonate/subscribe & hit. Breaking a compound down into. The relative ease with which the carbonates of. Zinc Carbonate Equation Thermal Decomposition.
From www.youtube.com
Calculating Ksp of Zinc Carbonate using electrode potentials YouTube Zinc Carbonate Equation Thermal Decomposition A metal carbonate that needs to be. Breaking a compound down into. Alkaline earth metal carbonates will decompose when heated making the metal oxide and carbon dioxide gas. The equation for the reaction is: In this video, i will explain the thermal decomposition of zinc carbonate/subscribe & hit. The group 2 carbonates break down (decompose) when they are heated to. Zinc Carbonate Equation Thermal Decomposition.
From www.researchgate.net
(PDF) Synthesis of Zno Nanoparticles by Thermal of Basic Zinc Carbonate Equation Thermal Decomposition Cuco 3 (s) → cuo (s) + co 2 (g) copper(ii) carbonate → copper(ii) oxide + carbon dioxide. In a thermal decomposition reaction, there is only one reactant, but two or more products. In this video, i will explain the thermal decomposition of zinc carbonate/subscribe & hit. The relative ease with which the carbonates of some of the less reactive. Zinc Carbonate Equation Thermal Decomposition.
From www.youtube.com
How to Balance Zn(OH)2 = ZnO + H2O (Zinc hydroxide YouTube Zinc Carbonate Equation Thermal Decomposition It describes and explains how. Zinc carbonate breaks up to form zinc oxide and carbon dioxide. A metal carbonate that needs to be. Thermal decomposition of zinc carbonate. In this video, i will explain the thermal decomposition of zinc carbonate/subscribe & hit. In a thermal decomposition reaction, there is only one reactant, but two or more products. Breaking a compound. Zinc Carbonate Equation Thermal Decomposition.
From www.slideserve.com
PPT What is a chemical reaction? PowerPoint Presentation, free Zinc Carbonate Equation Thermal Decomposition It describes and explains how. The equation for the reaction is: In this video, i will explain the thermal decomposition of zinc carbonate/subscribe & hit. Zinc carbonate breaks up to form zinc oxide and carbon dioxide. Alkaline earth metal carbonates will decompose when heated making the metal oxide and carbon dioxide gas. The relative ease with which the carbonates of. Zinc Carbonate Equation Thermal Decomposition.
From www.researchgate.net
(PDF) Thermal of zinc carbonate hydroxide Zinc Carbonate Equation Thermal Decomposition A metal carbonate that needs to be. The relative ease with which the carbonates of some of the less reactive metals are decomposed has been used in the extraction of these metals from ores that contain the metal as a carbonate, for example zinc carbonate (calamine). Alkaline earth metal carbonates will decompose when heated making the metal oxide and carbon. Zinc Carbonate Equation Thermal Decomposition.
From www.numerade.com
SOLVED The of zinc carbonate, ZnCO3(s), into zinc oxide Zinc Carbonate Equation Thermal Decomposition Zinc carbonate breaks up to form zinc oxide and carbon dioxide. In a thermal decomposition reaction, there is only one reactant, but two or more products. The equation for the reaction is: Thermal decomposition of zinc carbonate. Breaking a compound down into. A metal carbonate that needs to be. Alkaline earth metal carbonates will decompose when heated making the metal. Zinc Carbonate Equation Thermal Decomposition.
From www.slideserve.com
PPT What is a chemical reaction? PowerPoint Presentation, free Zinc Carbonate Equation Thermal Decomposition It describes and explains how. Cuco 3 (s) → cuo (s) + co 2 (g) copper(ii) carbonate → copper(ii) oxide + carbon dioxide. The group 2 carbonates break down (decompose) when they are heated to form the metal oxide and give off. In a thermal decomposition reaction, there is only one reactant, but two or more products. In this video,. Zinc Carbonate Equation Thermal Decomposition.
From www.onlinemathlearning.com
of Metal Compounds (solutions, examples, activities Zinc Carbonate Equation Thermal Decomposition Zinc carbonate breaks up to form zinc oxide and carbon dioxide. In this video, i will explain the thermal decomposition of zinc carbonate/subscribe & hit. The relative ease with which the carbonates of some of the less reactive metals are decomposed has been used in the extraction of these metals from ores that contain the metal as a carbonate, for. Zinc Carbonate Equation Thermal Decomposition.
From keystagewiki.com
Thermal Key Stage Wiki Zinc Carbonate Equation Thermal Decomposition It describes and explains how. In a thermal decomposition reaction, there is only one reactant, but two or more products. The equation for the reaction is: Breaking a compound down into. You could see the effect of the gas. In this video, i will explain the thermal decomposition of zinc carbonate/subscribe & hit. Thermal decomposition of zinc carbonate. Zinc carbonate. Zinc Carbonate Equation Thermal Decomposition.
From www.numerade.com
SOLVED The of zinc carbonate, \mathrm{ZnCO}_{3}(\mathrm Zinc Carbonate Equation Thermal Decomposition In this video, i will explain the thermal decomposition of zinc carbonate/subscribe & hit. Breaking a compound down into. A metal carbonate that needs to be. Thermal decomposition of zinc carbonate. The relative ease with which the carbonates of some of the less reactive metals are decomposed has been used in the extraction of these metals from ores that contain. Zinc Carbonate Equation Thermal Decomposition.