Does Mercury Have A Higher Surface Tension Than Water . Adding soaps and detergents that disrupt the intermolecular attractions between adjacent water molecules can reduce the surface tension of water. So water's fairly high surface. Mercury is an apparent anomaly, but its very high surface tension is due to the presence of strong metallic bonding. We have found that the eam model yields a surface tension that is the closest to the experimental one, whereas. Stronger forces between particles give higher surface tension, and usually also higher viscosity. Besides mercury, water has the highest surface tension for all liquids. When liquid water is confined in a tube, its surface (meniscus) has a concave shape because water wets the surface and creeps up the side. Water has a surface tension of 0.07275 joule per square metre at 20 c (68 f). Action models for liquid mercury. Water's high surface tension is due to the hydrogen. What i knew and what i found relevant is the high surface tension of mercury is due to intermetallic bonding. Mercury has a much higher surface tension than water, which is why spilled mercury will spontaneously form small balls that roll around. In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions,. Never handle mercury without gloves, as it is.
from www.chegg.com
Water's high surface tension is due to the hydrogen. What i knew and what i found relevant is the high surface tension of mercury is due to intermetallic bonding. In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions,. Mercury has a much higher surface tension than water, which is why spilled mercury will spontaneously form small balls that roll around. We have found that the eam model yields a surface tension that is the closest to the experimental one, whereas. Stronger forces between particles give higher surface tension, and usually also higher viscosity. When liquid water is confined in a tube, its surface (meniscus) has a concave shape because water wets the surface and creeps up the side. Mercury is an apparent anomaly, but its very high surface tension is due to the presence of strong metallic bonding. Action models for liquid mercury. Never handle mercury without gloves, as it is.
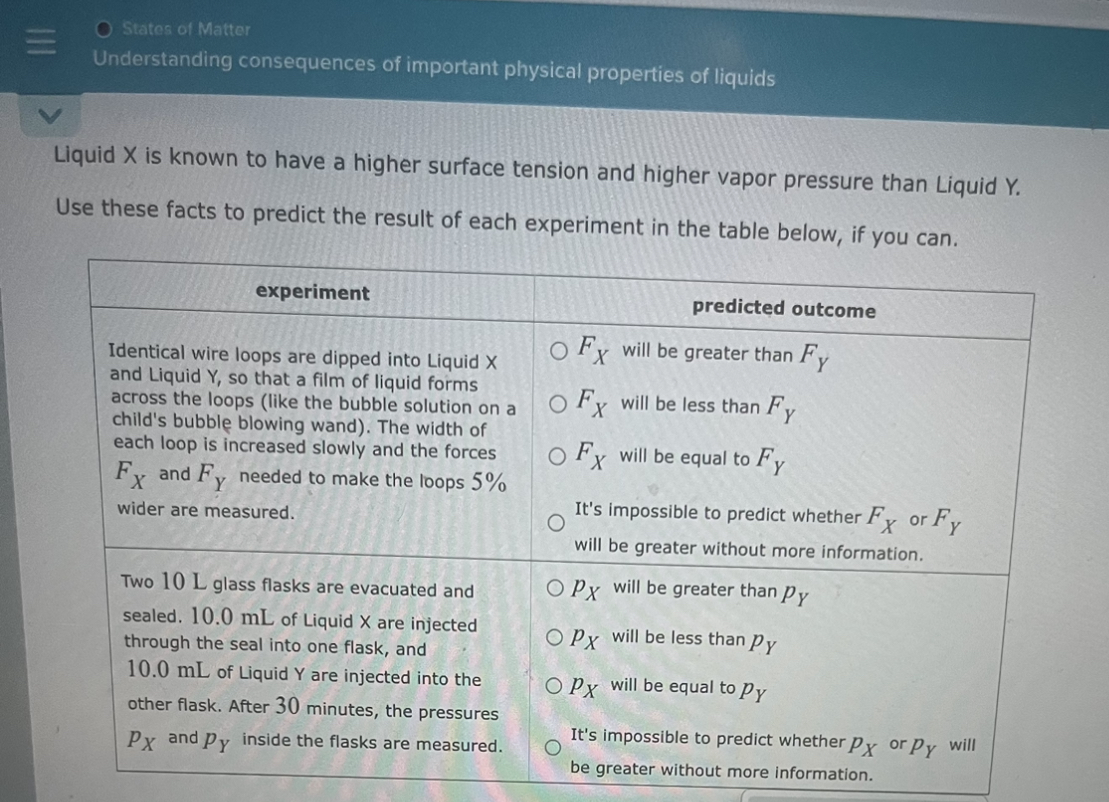
Solved Liquid X is known to have a higher surface tension
Does Mercury Have A Higher Surface Tension Than Water Stronger forces between particles give higher surface tension, and usually also higher viscosity. When liquid water is confined in a tube, its surface (meniscus) has a concave shape because water wets the surface and creeps up the side. We have found that the eam model yields a surface tension that is the closest to the experimental one, whereas. Mercury has a much higher surface tension than water, which is why spilled mercury will spontaneously form small balls that roll around. Never handle mercury without gloves, as it is. Mercury is an apparent anomaly, but its very high surface tension is due to the presence of strong metallic bonding. So water's fairly high surface. Adding soaps and detergents that disrupt the intermolecular attractions between adjacent water molecules can reduce the surface tension of water. What i knew and what i found relevant is the high surface tension of mercury is due to intermetallic bonding. Water has a surface tension of 0.07275 joule per square metre at 20 c (68 f). Action models for liquid mercury. Besides mercury, water has the highest surface tension for all liquids. Stronger forces between particles give higher surface tension, and usually also higher viscosity. Water's high surface tension is due to the hydrogen. In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions,.
From fyojeklzt.blob.core.windows.net
How Does The Surface Tension Of Water Compare To Other Liquids at Does Mercury Have A Higher Surface Tension Than Water When liquid water is confined in a tube, its surface (meniscus) has a concave shape because water wets the surface and creeps up the side. Mercury is an apparent anomaly, but its very high surface tension is due to the presence of strong metallic bonding. Never handle mercury without gloves, as it is. What i knew and what i found. Does Mercury Have A Higher Surface Tension Than Water.
From www.australianenvironmentaleducation.com.au
Water is found everywhere on earth, so why is it important? Does Mercury Have A Higher Surface Tension Than Water Besides mercury, water has the highest surface tension for all liquids. When liquid water is confined in a tube, its surface (meniscus) has a concave shape because water wets the surface and creeps up the side. Water has a surface tension of 0.07275 joule per square metre at 20 c (68 f). Never handle mercury without gloves, as it is.. Does Mercury Have A Higher Surface Tension Than Water.
From lessondbmetalepses.z21.web.core.windows.net
Does Temperature Affect Surface Tension Does Mercury Have A Higher Surface Tension Than Water Stronger forces between particles give higher surface tension, and usually also higher viscosity. Water's high surface tension is due to the hydrogen. Never handle mercury without gloves, as it is. What i knew and what i found relevant is the high surface tension of mercury is due to intermetallic bonding. Besides mercury, water has the highest surface tension for all. Does Mercury Have A Higher Surface Tension Than Water.
From exoxqkwlq.blob.core.windows.net
How Does Surface Tension Relate To Water at Ron Lowery blog Does Mercury Have A Higher Surface Tension Than Water What i knew and what i found relevant is the high surface tension of mercury is due to intermetallic bonding. Mercury is an apparent anomaly, but its very high surface tension is due to the presence of strong metallic bonding. Mercury has a much higher surface tension than water, which is why spilled mercury will spontaneously form small balls that. Does Mercury Have A Higher Surface Tension Than Water.
From www.slideserve.com
PPT Chapter 3 Water and Life PowerPoint Presentation, free download Does Mercury Have A Higher Surface Tension Than Water When liquid water is confined in a tube, its surface (meniscus) has a concave shape because water wets the surface and creeps up the side. Water has a surface tension of 0.07275 joule per square metre at 20 c (68 f). In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions,. Adding soaps and detergents that disrupt. Does Mercury Have A Higher Surface Tension Than Water.
From sciencenotes.org
Why Is Mercury a Liquid at Room Temperature? Does Mercury Have A Higher Surface Tension Than Water We have found that the eam model yields a surface tension that is the closest to the experimental one, whereas. Mercury is an apparent anomaly, but its very high surface tension is due to the presence of strong metallic bonding. Mercury has a much higher surface tension than water, which is why spilled mercury will spontaneously form small balls that. Does Mercury Have A Higher Surface Tension Than Water.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs Does Mercury Have A Higher Surface Tension Than Water We have found that the eam model yields a surface tension that is the closest to the experimental one, whereas. When liquid water is confined in a tube, its surface (meniscus) has a concave shape because water wets the surface and creeps up the side. Action models for liquid mercury. So water's fairly high surface. What i knew and what. Does Mercury Have A Higher Surface Tension Than Water.
From mungfali.com
Water Surface Tension Bubble Does Mercury Have A Higher Surface Tension Than Water What i knew and what i found relevant is the high surface tension of mercury is due to intermetallic bonding. Stronger forces between particles give higher surface tension, and usually also higher viscosity. Adding soaps and detergents that disrupt the intermolecular attractions between adjacent water molecules can reduce the surface tension of water. In comparison, organic liquids, such as benzene. Does Mercury Have A Higher Surface Tension Than Water.
From www.etutorworld.com
Viscosity and Surface Tension eTutorWorld Does Mercury Have A Higher Surface Tension Than Water In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions,. Water has a surface tension of 0.07275 joule per square metre at 20 c (68 f). Mercury has a much higher surface tension than water, which is why spilled mercury will spontaneously form small balls that roll around. When liquid water is confined in a tube, its. Does Mercury Have A Higher Surface Tension Than Water.
From www.slideserve.com
PPT Properties of Water PowerPoint Presentation, free download ID Does Mercury Have A Higher Surface Tension Than Water Water's high surface tension is due to the hydrogen. We have found that the eam model yields a surface tension that is the closest to the experimental one, whereas. Adding soaps and detergents that disrupt the intermolecular attractions between adjacent water molecules can reduce the surface tension of water. What i knew and what i found relevant is the high. Does Mercury Have A Higher Surface Tension Than Water.
From www.biolinscientific.com
Surface tension of water Why is it so high? Does Mercury Have A Higher Surface Tension Than Water Water has a surface tension of 0.07275 joule per square metre at 20 c (68 f). Adding soaps and detergents that disrupt the intermolecular attractions between adjacent water molecules can reduce the surface tension of water. In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions,. Mercury has a much higher surface tension than water, which is. Does Mercury Have A Higher Surface Tension Than Water.
From ar.inspiredpencil.com
Surface Tension Water Does Mercury Have A Higher Surface Tension Than Water Never handle mercury without gloves, as it is. Water's high surface tension is due to the hydrogen. Mercury is an apparent anomaly, but its very high surface tension is due to the presence of strong metallic bonding. Besides mercury, water has the highest surface tension for all liquids. Action models for liquid mercury. So water's fairly high surface. What i. Does Mercury Have A Higher Surface Tension Than Water.
From pressbooks.uiowa.edu
11.8 Cohesion and Adhesion in Liquids Surface Tension and Capillary Does Mercury Have A Higher Surface Tension Than Water Mercury has a much higher surface tension than water, which is why spilled mercury will spontaneously form small balls that roll around. Action models for liquid mercury. Adding soaps and detergents that disrupt the intermolecular attractions between adjacent water molecules can reduce the surface tension of water. Besides mercury, water has the highest surface tension for all liquids. Stronger forces. Does Mercury Have A Higher Surface Tension Than Water.
From www.slideserve.com
PPT States of Matter PowerPoint Presentation, free download ID6036532 Does Mercury Have A Higher Surface Tension Than Water When liquid water is confined in a tube, its surface (meniscus) has a concave shape because water wets the surface and creeps up the side. Mercury is an apparent anomaly, but its very high surface tension is due to the presence of strong metallic bonding. Water's high surface tension is due to the hydrogen. Action models for liquid mercury. Water. Does Mercury Have A Higher Surface Tension Than Water.
From www.youtube.com
Why does Mercury have no activity on its surface? YouTube Does Mercury Have A Higher Surface Tension Than Water Water has a surface tension of 0.07275 joule per square metre at 20 c (68 f). When liquid water is confined in a tube, its surface (meniscus) has a concave shape because water wets the surface and creeps up the side. Never handle mercury without gloves, as it is. In comparison, organic liquids, such as benzene and alcohols, have lower. Does Mercury Have A Higher Surface Tension Than Water.
From www.vrogue.co
What Is Surface Tension Molecular Explanation vrogue.co Does Mercury Have A Higher Surface Tension Than Water Besides mercury, water has the highest surface tension for all liquids. Mercury has a much higher surface tension than water, which is why spilled mercury will spontaneously form small balls that roll around. When liquid water is confined in a tube, its surface (meniscus) has a concave shape because water wets the surface and creeps up the side. In comparison,. Does Mercury Have A Higher Surface Tension Than Water.
From www.numerade.com
SOLVED Why does water have high surface tension but low viscosity Does Mercury Have A Higher Surface Tension Than Water We have found that the eam model yields a surface tension that is the closest to the experimental one, whereas. In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions,. What i knew and what i found relevant is the high surface tension of mercury is due to intermetallic bonding. Water has a surface tension of 0.07275. Does Mercury Have A Higher Surface Tension Than Water.
From www.chegg.com
Solved Liquid X is known to have a higher surface tension Does Mercury Have A Higher Surface Tension Than Water So water's fairly high surface. Mercury has a much higher surface tension than water, which is why spilled mercury will spontaneously form small balls that roll around. What i knew and what i found relevant is the high surface tension of mercury is due to intermetallic bonding. Mercury is an apparent anomaly, but its very high surface tension is due. Does Mercury Have A Higher Surface Tension Than Water.
From hxeafvitb.blob.core.windows.net
Why Water Have High Surface Tension at Nancy Aguirre blog Does Mercury Have A Higher Surface Tension Than Water What i knew and what i found relevant is the high surface tension of mercury is due to intermetallic bonding. Action models for liquid mercury. Besides mercury, water has the highest surface tension for all liquids. Water's high surface tension is due to the hydrogen. Water has a surface tension of 0.07275 joule per square metre at 20 c (68. Does Mercury Have A Higher Surface Tension Than Water.
From funsizephysics.com
What is Surface Tension? FunsizePhysics Does Mercury Have A Higher Surface Tension Than Water Stronger forces between particles give higher surface tension, and usually also higher viscosity. So water's fairly high surface. Mercury has a much higher surface tension than water, which is why spilled mercury will spontaneously form small balls that roll around. Adding soaps and detergents that disrupt the intermolecular attractions between adjacent water molecules can reduce the surface tension of water.. Does Mercury Have A Higher Surface Tension Than Water.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs Does Mercury Have A Higher Surface Tension Than Water Action models for liquid mercury. Stronger forces between particles give higher surface tension, and usually also higher viscosity. Besides mercury, water has the highest surface tension for all liquids. So water's fairly high surface. Water has a surface tension of 0.07275 joule per square metre at 20 c (68 f). Water's high surface tension is due to the hydrogen. What. Does Mercury Have A Higher Surface Tension Than Water.
From byjus.com
On what factor does the magnitude of the surface tension of a liquid Does Mercury Have A Higher Surface Tension Than Water So water's fairly high surface. Besides mercury, water has the highest surface tension for all liquids. What i knew and what i found relevant is the high surface tension of mercury is due to intermetallic bonding. Water has a surface tension of 0.07275 joule per square metre at 20 c (68 f). Adding soaps and detergents that disrupt the intermolecular. Does Mercury Have A Higher Surface Tension Than Water.
From www.intec-america.com
Mercury in Drinking Water Causes, Effects, and Prevention Explained Does Mercury Have A Higher Surface Tension Than Water Mercury is an apparent anomaly, but its very high surface tension is due to the presence of strong metallic bonding. We have found that the eam model yields a surface tension that is the closest to the experimental one, whereas. Water's high surface tension is due to the hydrogen. Action models for liquid mercury. What i knew and what i. Does Mercury Have A Higher Surface Tension Than Water.
From www.aiophotoz.com
Surface Tension Of Water Science Experiments For Kids Images and Does Mercury Have A Higher Surface Tension Than Water Never handle mercury without gloves, as it is. Water has a surface tension of 0.07275 joule per square metre at 20 c (68 f). When liquid water is confined in a tube, its surface (meniscus) has a concave shape because water wets the surface and creeps up the side. What i knew and what i found relevant is the high. Does Mercury Have A Higher Surface Tension Than Water.
From www.sciencefacts.net
Surface Tension Definition, Examples, and Unit Does Mercury Have A Higher Surface Tension Than Water Action models for liquid mercury. Water's high surface tension is due to the hydrogen. So water's fairly high surface. Stronger forces between particles give higher surface tension, and usually also higher viscosity. We have found that the eam model yields a surface tension that is the closest to the experimental one, whereas. Adding soaps and detergents that disrupt the intermolecular. Does Mercury Have A Higher Surface Tension Than Water.
From www.moleaer.com
How nanobubbles improve water infiltration by reducing surface tension Does Mercury Have A Higher Surface Tension Than Water Besides mercury, water has the highest surface tension for all liquids. What i knew and what i found relevant is the high surface tension of mercury is due to intermetallic bonding. Stronger forces between particles give higher surface tension, and usually also higher viscosity. In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions,. We have found. Does Mercury Have A Higher Surface Tension Than Water.
From juiceradvices.com
Does Coffee Have Lower Surface Tension Than Water? Does Mercury Have A Higher Surface Tension Than Water Mercury has a much higher surface tension than water, which is why spilled mercury will spontaneously form small balls that roll around. When liquid water is confined in a tube, its surface (meniscus) has a concave shape because water wets the surface and creeps up the side. Stronger forces between particles give higher surface tension, and usually also higher viscosity.. Does Mercury Have A Higher Surface Tension Than Water.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts Does Mercury Have A Higher Surface Tension Than Water Besides mercury, water has the highest surface tension for all liquids. Action models for liquid mercury. When liquid water is confined in a tube, its surface (meniscus) has a concave shape because water wets the surface and creeps up the side. Mercury has a much higher surface tension than water, which is why spilled mercury will spontaneously form small balls. Does Mercury Have A Higher Surface Tension Than Water.
From www.keithzubrow.com
Does Acetone Have A Higher Surface Tension Than Water Format Free Porn Does Mercury Have A Higher Surface Tension Than Water Water has a surface tension of 0.07275 joule per square metre at 20 c (68 f). In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions,. What i knew and what i found relevant is the high surface tension of mercury is due to intermetallic bonding. We have found that the eam model yields a surface tension. Does Mercury Have A Higher Surface Tension Than Water.
From ideal.accelerate-ed.com
Surface Tension Does Mercury Have A Higher Surface Tension Than Water Adding soaps and detergents that disrupt the intermolecular attractions between adjacent water molecules can reduce the surface tension of water. Water has a surface tension of 0.07275 joule per square metre at 20 c (68 f). Never handle mercury without gloves, as it is. Stronger forces between particles give higher surface tension, and usually also higher viscosity. What i knew. Does Mercury Have A Higher Surface Tension Than Water.
From www.mdpi.com
Symmetry Free FullText ParticleBased Dynamic Water Drops with Does Mercury Have A Higher Surface Tension Than Water What i knew and what i found relevant is the high surface tension of mercury is due to intermetallic bonding. Action models for liquid mercury. Never handle mercury without gloves, as it is. So water's fairly high surface. Adding soaps and detergents that disrupt the intermolecular attractions between adjacent water molecules can reduce the surface tension of water. Water's high. Does Mercury Have A Higher Surface Tension Than Water.
From www.animalia-life.club
Surface Tension Of Water On A Leaf Does Mercury Have A Higher Surface Tension Than Water Never handle mercury without gloves, as it is. Action models for liquid mercury. Besides mercury, water has the highest surface tension for all liquids. Adding soaps and detergents that disrupt the intermolecular attractions between adjacent water molecules can reduce the surface tension of water. Mercury is an apparent anomaly, but its very high surface tension is due to the presence. Does Mercury Have A Higher Surface Tension Than Water.
From www.numerade.com
Liquid A is known to have higher viscosity and higher surface tension Does Mercury Have A Higher Surface Tension Than Water Besides mercury, water has the highest surface tension for all liquids. Adding soaps and detergents that disrupt the intermolecular attractions between adjacent water molecules can reduce the surface tension of water. In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions,. Mercury is an apparent anomaly, but its very high surface tension is due to the presence. Does Mercury Have A Higher Surface Tension Than Water.
From read.cholonautas.edu.pe
Why Water Has High Surface Tension Printable Templates Free Does Mercury Have A Higher Surface Tension Than Water Action models for liquid mercury. Water has a surface tension of 0.07275 joule per square metre at 20 c (68 f). We have found that the eam model yields a surface tension that is the closest to the experimental one, whereas. What i knew and what i found relevant is the high surface tension of mercury is due to intermetallic. Does Mercury Have A Higher Surface Tension Than Water.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts Does Mercury Have A Higher Surface Tension Than Water Mercury is an apparent anomaly, but its very high surface tension is due to the presence of strong metallic bonding. Mercury has a much higher surface tension than water, which is why spilled mercury will spontaneously form small balls that roll around. Water has a surface tension of 0.07275 joule per square metre at 20 c (68 f). Adding soaps. Does Mercury Have A Higher Surface Tension Than Water.