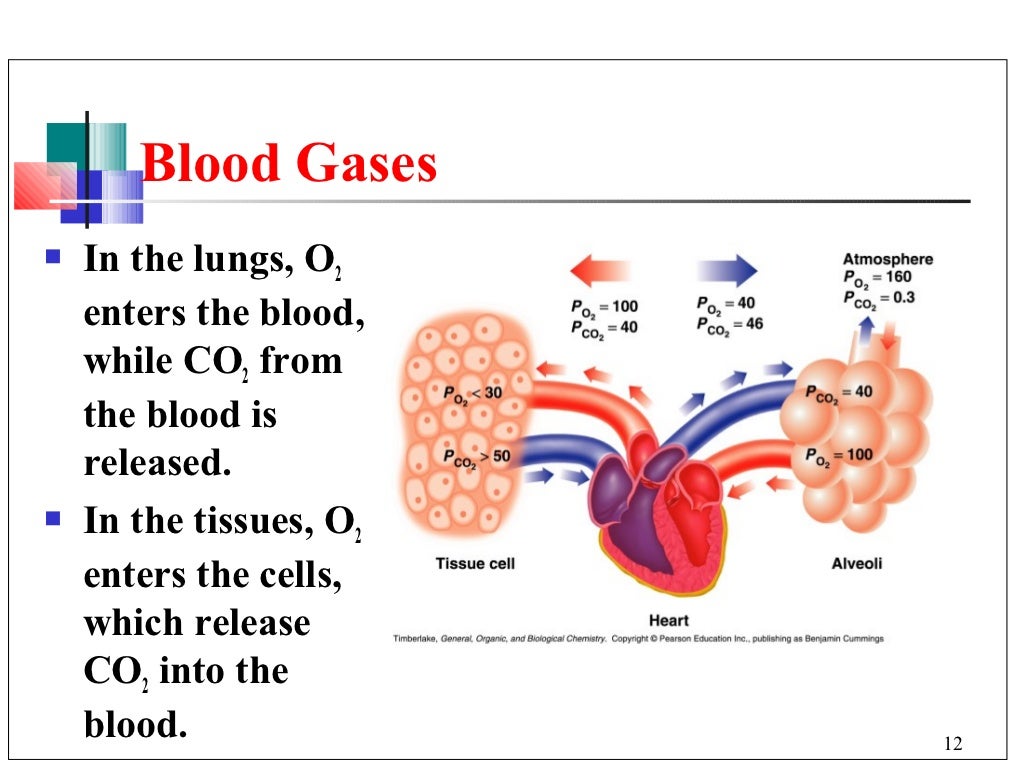
Why Is Blood A Buffer . Our blood is buffered to maintain a ph of 7.35 to 7.45 that. Buffers resist changes in the ph of a solution when hydrogen ions or hydroxide are added (or removed). Buffer solutions are essential components of all living organisms. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. In this system, gaseous metabolic waste carbon dioxide reacts. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain. Buffers dissociate in solution and neutralize extra hydrogen ions or hydroxide.
from www.slideshare.net
Buffers resist changes in the ph of a solution when hydrogen ions or hydroxide are added (or removed). Buffer solutions are essential components of all living organisms. In this system, gaseous metabolic waste carbon dioxide reacts. Our blood is buffered to maintain a ph of 7.35 to 7.45 that. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. Buffers dissociate in solution and neutralize extra hydrogen ions or hydroxide.
Buffer in the blood
Why Is Blood A Buffer Our blood is buffered to maintain a ph of 7.35 to 7.45 that. In this system, gaseous metabolic waste carbon dioxide reacts. Our blood is buffered to maintain a ph of 7.35 to 7.45 that. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. Buffers dissociate in solution and neutralize extra hydrogen ions or hydroxide. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain. Buffers resist changes in the ph of a solution when hydrogen ions or hydroxide are added (or removed). Buffer solutions are essential components of all living organisms.
From www.slideshare.net
Buffer in the blood Why Is Blood A Buffer Buffer solutions are essential components of all living organisms. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain. Buffers dissociate in solution and neutralize extra hydrogen ions or hydroxide. Our blood is buffered to maintain a ph of 7.35 to 7.45 that. In this system,. Why Is Blood A Buffer.
From www.slideserve.com
PPT Renal Physiology PowerPoint Presentation, free download ID5632772 Why Is Blood A Buffer Buffers dissociate in solution and neutralize extra hydrogen ions or hydroxide. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain. Buffer solutions. Why Is Blood A Buffer.
From iastate.pressbooks.pub
Properties of Blood as a Buffer and Blood Glucose A Mixed Course Why Is Blood A Buffer In this system, gaseous metabolic waste carbon dioxide reacts. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain. Our blood is buffered to maintain a ph of 7.35 to 7.45 that. A variety of buffering systems permits blood and other bodily fluids to maintain a. Why Is Blood A Buffer.
From beta.learner.org
The Buffer System in the Blood (animation) Annenberg Learner Why Is Blood A Buffer Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain. In this system, gaseous metabolic waste carbon dioxide reacts. Buffers dissociate in solution and neutralize extra hydrogen ions or hydroxide. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph. Why Is Blood A Buffer.
From en.ppt-online.org
Blood biochemistry online presentation Why Is Blood A Buffer In this system, gaseous metabolic waste carbon dioxide reacts. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. Our blood is buffered to maintain a ph of 7.35 to 7.45 that. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate. Why Is Blood A Buffer.
From ppt-online.org
Disorders of metabolism. (Subject 9) презентация онлайн Why Is Blood A Buffer Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain. Our blood is buffered to maintain a ph of 7.35 to 7.45 that. Buffers resist changes in the ph of a solution when hydrogen ions or hydroxide are added (or removed). Buffer solutions are essential components. Why Is Blood A Buffer.
From facts.net
20 Fascinating Facts About Blood Buffer Why Is Blood A Buffer Our blood is buffered to maintain a ph of 7.35 to 7.45 that. Buffer solutions are essential components of all living organisms. In this system, gaseous metabolic waste carbon dioxide reacts. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain. A variety of buffering systems. Why Is Blood A Buffer.
From www.youtube.com
Buffer action in the blood YouTube Why Is Blood A Buffer Our blood is buffered to maintain a ph of 7.35 to 7.45 that. Buffer solutions are essential components of all living organisms. Buffers resist changes in the ph of a solution when hydrogen ions or hydroxide are added (or removed). Buffers dissociate in solution and neutralize extra hydrogen ions or hydroxide. Human blood contains a buffer of carbonic acid (h2co3. Why Is Blood A Buffer.
From www.slideserve.com
PPT Biological Buffers PowerPoint Presentation, free download ID Why Is Blood A Buffer In this system, gaseous metabolic waste carbon dioxide reacts. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain. Our blood is buffered to maintain a ph of 7.35 to 7.45 that. A variety of buffering systems permits blood and other bodily fluids to maintain a. Why Is Blood A Buffer.
From www.slideserve.com
PPT Physiological system of blood. Functional importance of blood Why Is Blood A Buffer Buffers resist changes in the ph of a solution when hydrogen ions or hydroxide are added (or removed). Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain. Our blood is buffered to maintain a ph of 7.35 to 7.45 that. A variety of buffering systems. Why Is Blood A Buffer.
From www.slideshare.net
Buffer in the blood Why Is Blood A Buffer Buffer solutions are essential components of all living organisms. In this system, gaseous metabolic waste carbon dioxide reacts. Buffers dissociate in solution and neutralize extra hydrogen ions or hydroxide. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain. Our blood is buffered to maintain a. Why Is Blood A Buffer.
From www.slideserve.com
PPT Salts in Solution Buffers PowerPoint Presentation, free download Why Is Blood A Buffer A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. In this system, gaseous metabolic waste carbon dioxide reacts. Our blood is buffered to maintain a ph of 7.35 to 7.45 that. Buffers dissociate in solution and neutralize extra hydrogen ions or hydroxide. Human blood contains a. Why Is Blood A Buffer.
From www.slideshare.net
Blood buffer system Why Is Blood A Buffer A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. In this system, gaseous metabolic waste carbon dioxide reacts. Buffers resist changes in the ph of a solution when hydrogen ions or hydroxide are added (or removed). Our blood is buffered to maintain a ph of 7.35. Why Is Blood A Buffer.
From www.slideserve.com
PPT ACID BASE PHYSIOLOGY PowerPoint Presentation, free download Why Is Blood A Buffer Buffers resist changes in the ph of a solution when hydrogen ions or hydroxide are added (or removed). Buffer solutions are essential components of all living organisms. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. Buffers dissociate in solution and neutralize extra hydrogen ions or. Why Is Blood A Buffer.
From www.youtube.com
Blood Buffer System Chemical Buffer System Bicarbonate Buffer System Why Is Blood A Buffer Our blood is buffered to maintain a ph of 7.35 to 7.45 that. Buffers dissociate in solution and neutralize extra hydrogen ions or hydroxide. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain. A variety of buffering systems permits blood and other bodily fluids to. Why Is Blood A Buffer.
From www.slideserve.com
PPT Blood Buffers PowerPoint Presentation, free download ID2730981 Why Is Blood A Buffer Buffer solutions are essential components of all living organisms. Our blood is buffered to maintain a ph of 7.35 to 7.45 that. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain. In this system, gaseous metabolic waste carbon dioxide reacts. A variety of buffering systems. Why Is Blood A Buffer.
From www.slideserve.com
PPT MLAB 2401 Clinical Chemistry Keri BrophyMartinez PowerPoint Why Is Blood A Buffer A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. Our blood is buffered to maintain a ph of 7.35 to 7.45 that. Buffers dissociate in solution and neutralize extra hydrogen ions or hydroxide. Buffers resist changes in the ph of a solution when hydrogen ions or. Why Is Blood A Buffer.
From www.slideshare.net
Blood buffers and their role in regulation of homeostasis PDF Why Is Blood A Buffer A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. In this system, gaseous metabolic waste carbon dioxide reacts. Buffer solutions are essential components of all living organisms. Our blood is buffered to maintain a ph of 7.35 to 7.45 that. Human blood contains a buffer of. Why Is Blood A Buffer.
From www.slideserve.com
PPT MLAB 2401 Clinical Chemistry Keri BrophyMartinez PowerPoint Why Is Blood A Buffer Our blood is buffered to maintain a ph of 7.35 to 7.45 that. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. Buffers dissociate in solution and neutralize extra hydrogen ions or hydroxide. Buffers resist changes in the ph of a solution when hydrogen ions or. Why Is Blood A Buffer.
From www.slideserve.com
PPT Buffers and Titrations PowerPoint Presentation, free download Why Is Blood A Buffer A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. In this system, gaseous metabolic waste carbon dioxide reacts. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain. Buffer solutions are essential. Why Is Blood A Buffer.
From www.slideserve.com
PPT Buffers and Titrations PowerPoint Presentation, free download Why Is Blood A Buffer Buffers dissociate in solution and neutralize extra hydrogen ions or hydroxide. Our blood is buffered to maintain a ph of 7.35 to 7.45 that. In this system, gaseous metabolic waste carbon dioxide reacts. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain. A variety of. Why Is Blood A Buffer.
From www.slideserve.com
PPT Buffers and Titrations PowerPoint Presentation, free download Why Is Blood A Buffer In this system, gaseous metabolic waste carbon dioxide reacts. Buffers dissociate in solution and neutralize extra hydrogen ions or hydroxide. Buffers resist changes in the ph of a solution when hydrogen ions or hydroxide are added (or removed). A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of. Why Is Blood A Buffer.
From www.slideserve.com
PPT General, Organic, and Biochemistry, 7e PowerPoint Presentation Why Is Blood A Buffer Buffers dissociate in solution and neutralize extra hydrogen ions or hydroxide. Buffer solutions are essential components of all living organisms. Buffers resist changes in the ph of a solution when hydrogen ions or hydroxide are added (or removed). A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of. Why Is Blood A Buffer.
From www.slideserve.com
PPT Buffers in Blood. Acidosis and Alkalosis. PowerPoint Presentation Why Is Blood A Buffer Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain. In this system, gaseous metabolic waste carbon dioxide reacts. Our blood is buffered to maintain a ph of 7.35 to 7.45 that. Buffer solutions are essential components of all living organisms. A variety of buffering systems. Why Is Blood A Buffer.
From www.pinterest.com
Physiology Blood Buffer System Behrouz Human body facts Why Is Blood A Buffer Buffers resist changes in the ph of a solution when hydrogen ions or hydroxide are added (or removed). In this system, gaseous metabolic waste carbon dioxide reacts. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. Human blood contains a buffer of carbonic acid (h2co3 h. Why Is Blood A Buffer.
From www.slideserve.com
PPT MLAB 2401 Clinical Chemistry Keri BrophyMartinez PowerPoint Why Is Blood A Buffer Buffer solutions are essential components of all living organisms. Our blood is buffered to maintain a ph of 7.35 to 7.45 that. Buffers dissociate in solution and neutralize extra hydrogen ions or hydroxide. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain. Buffers resist changes. Why Is Blood A Buffer.
From www.slideserve.com
PPT pH and Buffers PowerPoint Presentation, free download ID6910668 Why Is Blood A Buffer Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain. Buffers resist changes in the ph of a solution when hydrogen ions or hydroxide are added (or removed). Buffer solutions are essential components of all living organisms. Our blood is buffered to maintain a ph of. Why Is Blood A Buffer.
From www.slideserve.com
PPT Blood Buffers PowerPoint Presentation, free download ID5687657 Why Is Blood A Buffer Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain. Buffer solutions are essential components of all living organisms. Our blood is buffered to maintain a ph of 7.35 to 7.45 that. A variety of buffering systems permits blood and other bodily fluids to maintain a. Why Is Blood A Buffer.
From www.slideserve.com
PPT Buffers in Blood. Acidosis and Alkalosis. PowerPoint Presentation Why Is Blood A Buffer Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain. Buffers dissociate in solution and neutralize extra hydrogen ions or hydroxide. Buffers resist changes in the ph of a solution when hydrogen ions or hydroxide are added (or removed). In this system, gaseous metabolic waste carbon. Why Is Blood A Buffer.
From philschatz.com
AcidBase Balance · Anatomy and Physiology Why Is Blood A Buffer Buffers resist changes in the ph of a solution when hydrogen ions or hydroxide are added (or removed). Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain. Buffers dissociate in solution and neutralize extra hydrogen ions or hydroxide. In this system, gaseous metabolic waste carbon. Why Is Blood A Buffer.
From www.slideserve.com
PPT Buffers in Blood. Acidosis and Alkalosis. PowerPoint Presentation Why Is Blood A Buffer Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. Buffers dissociate in solution and neutralize extra hydrogen ions or hydroxide. Buffer solutions. Why Is Blood A Buffer.
From www.youtube.com
Biochemistry acid, base, and buffer system blood buffer YouTube Why Is Blood A Buffer Our blood is buffered to maintain a ph of 7.35 to 7.45 that. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations.. Why Is Blood A Buffer.
From www.slideserve.com
PPT Salts in Solution Buffers PowerPoint Presentation, free download Why Is Blood A Buffer Buffers dissociate in solution and neutralize extra hydrogen ions or hydroxide. Buffers resist changes in the ph of a solution when hydrogen ions or hydroxide are added (or removed). Our blood is buffered to maintain a ph of 7.35 to 7.45 that. Buffer solutions are essential components of all living organisms. In this system, gaseous metabolic waste carbon dioxide reacts.. Why Is Blood A Buffer.
From www.slideserve.com
PPT Buffers in Blood. Acidosis and Alkalosis. PowerPoint Presentation Why Is Blood A Buffer Buffers dissociate in solution and neutralize extra hydrogen ions or hydroxide. Buffer solutions are essential components of all living organisms. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. In this system, gaseous metabolic waste carbon dioxide reacts. Human blood contains a buffer of carbonic acid. Why Is Blood A Buffer.
From www.slideserve.com
PPT Buffers and Salts PowerPoint Presentation, free download ID5405242 Why Is Blood A Buffer Buffers dissociate in solution and neutralize extra hydrogen ions or hydroxide. Buffer solutions are essential components of all living organisms. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph. Why Is Blood A Buffer.