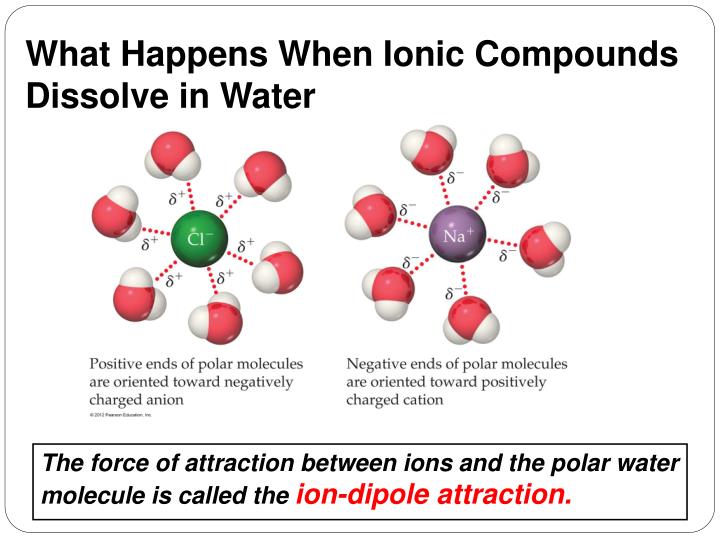
Which Substances Cannot Dissolve In Water And Why . They are described as hydrophobic, or water fearing. Nonpolar molecules, such as those found in grease or oil, do not dissolve in. Similarly, we find that polar solutes such as methanol, ethanol, and isopropanol are highly soluble in water. Insoluble substance is the substance which is not dissolved in a specific dissolving media. Make a list of substances that you know dissolve in water. Water typically dissolves most ionic compounds and polar molecules. When put into polar environments, such as water, nonpolar. Thus, halogenated organic compounds do not dissolve well in water. Metals, ionic compounds, molecular compounds (polar, non. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. Nonpolar molecules do not dissolve easily in water. When a solute dissolves in water, the process is referred to as hydration.
from www.slideserve.com
Nonpolar molecules, such as those found in grease or oil, do not dissolve in. When put into polar environments, such as water, nonpolar. Make a list of substances that you know dissolve in water. When a solute dissolves in water, the process is referred to as hydration. Water typically dissolves most ionic compounds and polar molecules. Nonpolar molecules do not dissolve easily in water. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. Similarly, we find that polar solutes such as methanol, ethanol, and isopropanol are highly soluble in water. Metals, ionic compounds, molecular compounds (polar, non. Insoluble substance is the substance which is not dissolved in a specific dissolving media.
PPT UNIT 5 PowerPoint Presentation ID2276550
Which Substances Cannot Dissolve In Water And Why Thus, halogenated organic compounds do not dissolve well in water. Nonpolar molecules, such as those found in grease or oil, do not dissolve in. Similarly, we find that polar solutes such as methanol, ethanol, and isopropanol are highly soluble in water. Insoluble substance is the substance which is not dissolved in a specific dissolving media. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. Make a list of substances that you know dissolve in water. When a solute dissolves in water, the process is referred to as hydration. Metals, ionic compounds, molecular compounds (polar, non. When put into polar environments, such as water, nonpolar. Nonpolar molecules do not dissolve easily in water. Thus, halogenated organic compounds do not dissolve well in water. Water typically dissolves most ionic compounds and polar molecules. They are described as hydrophobic, or water fearing.
From brainly.in
the substance that will not dissolve in water Brainly.in Which Substances Cannot Dissolve In Water And Why When put into polar environments, such as water, nonpolar. When a solute dissolves in water, the process is referred to as hydration. Water typically dissolves most ionic compounds and polar molecules. Nonpolar molecules, such as those found in grease or oil, do not dissolve in. Water, which not only dissolves many compounds but also dissolves more substances than any other. Which Substances Cannot Dissolve In Water And Why.
From www.science-sparks.com
Which solids dissolve in water Which Substances Cannot Dissolve In Water And Why When a solute dissolves in water, the process is referred to as hydration. Make a list of substances that you know dissolve in water. Insoluble substance is the substance which is not dissolved in a specific dissolving media. Water typically dissolves most ionic compounds and polar molecules. They are described as hydrophobic, or water fearing. Nonpolar molecules, such as those. Which Substances Cannot Dissolve In Water And Why.
From www.gauthmath.com
Solved What is an electrolyte? a substance that cannot dissolve in water a substance that Which Substances Cannot Dissolve In Water And Why Thus, halogenated organic compounds do not dissolve well in water. Nonpolar molecules, such as those found in grease or oil, do not dissolve in. Insoluble substance is the substance which is not dissolved in a specific dissolving media. Water typically dissolves most ionic compounds and polar molecules. Similarly, we find that polar solutes such as methanol, ethanol, and isopropanol are. Which Substances Cannot Dissolve In Water And Why.
From slideplayer.com
Solutions, Solvents, and Solutes ppt download Which Substances Cannot Dissolve In Water And Why Insoluble substance is the substance which is not dissolved in a specific dissolving media. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. When put into polar environments, such as water, nonpolar. Nonpolar molecules do not dissolve easily in water. Make a list of substances that you know. Which Substances Cannot Dissolve In Water And Why.
From www.numerade.com
SOLVED Which statement is true about dissolving? All solids and liquid substances can dissolve Which Substances Cannot Dissolve In Water And Why They are described as hydrophobic, or water fearing. Metals, ionic compounds, molecular compounds (polar, non. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. When a solute dissolves in water, the process is referred to as hydration. Nonpolar molecules do not dissolve easily in water. Thus, halogenated organic. Which Substances Cannot Dissolve In Water And Why.
From www.slideserve.com
PPT Unit 6 TOXINS Solutions & PowerPoint Presentation ID1999793 Which Substances Cannot Dissolve In Water And Why Nonpolar molecules do not dissolve easily in water. Metals, ionic compounds, molecular compounds (polar, non. They are described as hydrophobic, or water fearing. Nonpolar molecules, such as those found in grease or oil, do not dissolve in. Thus, halogenated organic compounds do not dissolve well in water. When put into polar environments, such as water, nonpolar. Insoluble substance is the. Which Substances Cannot Dissolve In Water And Why.
From www.numerade.com
SOLVED Five different substances are given to you to be dissolved in water. Which substances Which Substances Cannot Dissolve In Water And Why They are described as hydrophobic, or water fearing. When put into polar environments, such as water, nonpolar. Similarly, we find that polar solutes such as methanol, ethanol, and isopropanol are highly soluble in water. Metals, ionic compounds, molecular compounds (polar, non. Nonpolar molecules do not dissolve easily in water. Nonpolar molecules, such as those found in grease or oil, do. Which Substances Cannot Dissolve In Water And Why.
From www.youtube.com
Why oil does not dissolve in water Polarity of water YouTube Which Substances Cannot Dissolve In Water And Why Make a list of substances that you know dissolve in water. Nonpolar molecules do not dissolve easily in water. They are described as hydrophobic, or water fearing. Metals, ionic compounds, molecular compounds (polar, non. When put into polar environments, such as water, nonpolar. Nonpolar molecules, such as those found in grease or oil, do not dissolve in. Insoluble substance is. Which Substances Cannot Dissolve In Water And Why.
From sciencing.com
Substances That Won't Dissolve in Water Sciencing Which Substances Cannot Dissolve In Water And Why Insoluble substance is the substance which is not dissolved in a specific dissolving media. When put into polar environments, such as water, nonpolar. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. Nonpolar molecules, such as those found in grease or oil, do not dissolve in. When a. Which Substances Cannot Dissolve In Water And Why.
From slidetodoc.com
HOW AND WHY DO SUBSTANCES DISSOLVE IN WATER Which Substances Cannot Dissolve In Water And Why Thus, halogenated organic compounds do not dissolve well in water. They are described as hydrophobic, or water fearing. When put into polar environments, such as water, nonpolar. Metals, ionic compounds, molecular compounds (polar, non. Insoluble substance is the substance which is not dissolved in a specific dissolving media. Water typically dissolves most ionic compounds and polar molecules. Nonpolar molecules do. Which Substances Cannot Dissolve In Water And Why.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID2736092 Which Substances Cannot Dissolve In Water And Why When put into polar environments, such as water, nonpolar. Thus, halogenated organic compounds do not dissolve well in water. Nonpolar molecules, such as those found in grease or oil, do not dissolve in. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. Insoluble substance is the substance which. Which Substances Cannot Dissolve In Water And Why.
From shaunmwilliams.com
Chapter 3 Presentation Which Substances Cannot Dissolve In Water And Why Metals, ionic compounds, molecular compounds (polar, non. Thus, halogenated organic compounds do not dissolve well in water. Nonpolar molecules, such as those found in grease or oil, do not dissolve in. Insoluble substance is the substance which is not dissolved in a specific dissolving media. Similarly, we find that polar solutes such as methanol, ethanol, and isopropanol are highly soluble. Which Substances Cannot Dissolve In Water And Why.
From www.chegg.com
Solved 44. Substances which do not dissolve in water are Which Substances Cannot Dissolve In Water And Why Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. When put into polar environments, such as water, nonpolar. When a solute dissolves in water, the process is referred to as hydration. Water typically dissolves most ionic compounds and polar molecules. Metals, ionic compounds, molecular compounds (polar, non. Make. Which Substances Cannot Dissolve In Water And Why.
From www.numerade.com
SOLVEDSubstances that dissolve in water generally do not dissolve in benzene. Some substances Which Substances Cannot Dissolve In Water And Why Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. Metals, ionic compounds, molecular compounds (polar, non. Insoluble substance is the substance which is not dissolved in a specific dissolving media. They are described as hydrophobic, or water fearing. Nonpolar molecules do not dissolve easily in water. Water typically. Which Substances Cannot Dissolve In Water And Why.
From www.slideserve.com
PPT Solutions PowerPoint Presentation ID6888319 Which Substances Cannot Dissolve In Water And Why Water typically dissolves most ionic compounds and polar molecules. Nonpolar molecules do not dissolve easily in water. They are described as hydrophobic, or water fearing. Metals, ionic compounds, molecular compounds (polar, non. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. When put into polar environments, such as. Which Substances Cannot Dissolve In Water And Why.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation ID2276550 Which Substances Cannot Dissolve In Water And Why Nonpolar molecules do not dissolve easily in water. Thus, halogenated organic compounds do not dissolve well in water. When put into polar environments, such as water, nonpolar. Make a list of substances that you know dissolve in water. Nonpolar molecules, such as those found in grease or oil, do not dissolve in. Water, which not only dissolves many compounds but. Which Substances Cannot Dissolve In Water And Why.
From wou.edu
CH104 Chapter 7 Solutions Chemistry Which Substances Cannot Dissolve In Water And Why When a solute dissolves in water, the process is referred to as hydration. Water typically dissolves most ionic compounds and polar molecules. Nonpolar molecules, such as those found in grease or oil, do not dissolve in. Metals, ionic compounds, molecular compounds (polar, non. Insoluble substance is the substance which is not dissolved in a specific dissolving media. When put into. Which Substances Cannot Dissolve In Water And Why.
From www.bbc.co.uk
Which materials dissolve in water? BBC Bitesize Which Substances Cannot Dissolve In Water And Why Nonpolar molecules do not dissolve easily in water. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. Make a list of substances that you know dissolve in water. When put into polar environments, such as water, nonpolar. Nonpolar molecules, such as those found in grease or oil, do. Which Substances Cannot Dissolve In Water And Why.
From slideplayer.com
UNIT 1 Foundations of Biology ppt download Which Substances Cannot Dissolve In Water And Why Make a list of substances that you know dissolve in water. Thus, halogenated organic compounds do not dissolve well in water. Metals, ionic compounds, molecular compounds (polar, non. When put into polar environments, such as water, nonpolar. Nonpolar molecules, such as those found in grease or oil, do not dissolve in. When a solute dissolves in water, the process is. Which Substances Cannot Dissolve In Water And Why.
From www.science-sparks.com
Which Solids Dissolve In Water Cool Science for Kids Which Substances Cannot Dissolve In Water And Why When a solute dissolves in water, the process is referred to as hydration. Water typically dissolves most ionic compounds and polar molecules. Metals, ionic compounds, molecular compounds (polar, non. They are described as hydrophobic, or water fearing. Nonpolar molecules, such as those found in grease or oil, do not dissolve in. Similarly, we find that polar solutes such as methanol,. Which Substances Cannot Dissolve In Water And Why.
From www.slideserve.com
PPT Chemistry of Life PowerPoint Presentation, free download ID9217796 Which Substances Cannot Dissolve In Water And Why Insoluble substance is the substance which is not dissolved in a specific dissolving media. Nonpolar molecules, such as those found in grease or oil, do not dissolve in. Nonpolar molecules do not dissolve easily in water. They are described as hydrophobic, or water fearing. When a solute dissolves in water, the process is referred to as hydration. Metals, ionic compounds,. Which Substances Cannot Dissolve In Water And Why.
From www.slideserve.com
PPT Introduction to cells PowerPoint Presentation, free download ID1713490 Which Substances Cannot Dissolve In Water And Why Metals, ionic compounds, molecular compounds (polar, non. When put into polar environments, such as water, nonpolar. They are described as hydrophobic, or water fearing. Nonpolar molecules do not dissolve easily in water. Make a list of substances that you know dissolve in water. Similarly, we find that polar solutes such as methanol, ethanol, and isopropanol are highly soluble in water.. Which Substances Cannot Dissolve In Water And Why.
From www.youtube.com
Why do some substances dissolve in water and others do not? YouTube Which Substances Cannot Dissolve In Water And Why Insoluble substance is the substance which is not dissolved in a specific dissolving media. When put into polar environments, such as water, nonpolar. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. When a solute dissolves in water, the process is referred to as hydration. Metals, ionic compounds,. Which Substances Cannot Dissolve In Water And Why.
From www.slideserve.com
PPT Chemistry of Life PowerPoint Presentation ID5875746 Which Substances Cannot Dissolve In Water And Why When a solute dissolves in water, the process is referred to as hydration. When put into polar environments, such as water, nonpolar. Similarly, we find that polar solutes such as methanol, ethanol, and isopropanol are highly soluble in water. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent.. Which Substances Cannot Dissolve In Water And Why.
From byjus.com
Which among the following things do not dissolve in water? Which Substances Cannot Dissolve In Water And Why Thus, halogenated organic compounds do not dissolve well in water. Water typically dissolves most ionic compounds and polar molecules. When a solute dissolves in water, the process is referred to as hydration. Make a list of substances that you know dissolve in water. Nonpolar molecules do not dissolve easily in water. When put into polar environments, such as water, nonpolar.. Which Substances Cannot Dissolve In Water And Why.
From www.youtube.com
Which substances dissolve in water? YouTube Which Substances Cannot Dissolve In Water And Why Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. Similarly, we find that polar solutes such as methanol, ethanol, and isopropanol are highly soluble in water. They are described as hydrophobic, or water fearing. Insoluble substance is the substance which is not dissolved in a specific dissolving media.. Which Substances Cannot Dissolve In Water And Why.
From www.slideserve.com
PPT Solutes, Solutions and Solvents PowerPoint Presentation, free download ID2528981 Which Substances Cannot Dissolve In Water And Why Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. Water typically dissolves most ionic compounds and polar molecules. They are described as hydrophobic, or water fearing. Metals, ionic compounds, molecular compounds (polar, non. Thus, halogenated organic compounds do not dissolve well in water. Similarly, we find that polar. Which Substances Cannot Dissolve In Water And Why.
From publicaffairsworld.com
what dissolves in water science project Which Substances Cannot Dissolve In Water And Why Insoluble substance is the substance which is not dissolved in a specific dissolving media. Thus, halogenated organic compounds do not dissolve well in water. When a solute dissolves in water, the process is referred to as hydration. They are described as hydrophobic, or water fearing. When put into polar environments, such as water, nonpolar. Nonpolar molecules, such as those found. Which Substances Cannot Dissolve In Water And Why.
From www.chegg.com
Solved Classify which compounds will dissolve in water and Which Substances Cannot Dissolve In Water And Why Make a list of substances that you know dissolve in water. When a solute dissolves in water, the process is referred to as hydration. When put into polar environments, such as water, nonpolar. Insoluble substance is the substance which is not dissolved in a specific dissolving media. Water typically dissolves most ionic compounds and polar molecules. Similarly, we find that. Which Substances Cannot Dissolve In Water And Why.
From www.youtube.com
Why are ionic compounds soluble in water? YouTube Which Substances Cannot Dissolve In Water And Why Similarly, we find that polar solutes such as methanol, ethanol, and isopropanol are highly soluble in water. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. When a solute dissolves in water, the process is referred to as hydration. Metals, ionic compounds, molecular compounds (polar, non. When put. Which Substances Cannot Dissolve In Water And Why.
From www.youtube.com
DYK Water can dissolve more substances than any other liquid because shorts YouTube Which Substances Cannot Dissolve In Water And Why Nonpolar molecules do not dissolve easily in water. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. Metals, ionic compounds, molecular compounds (polar, non. Insoluble substance is the substance which is not dissolved in a specific dissolving media. Similarly, we find that polar solutes such as methanol, ethanol,. Which Substances Cannot Dissolve In Water And Why.
From www.slideserve.com
PPT Water, Water, Everywhere (Ch. 3) PowerPoint Presentation, free download ID5799088 Which Substances Cannot Dissolve In Water And Why Make a list of substances that you know dissolve in water. Metals, ionic compounds, molecular compounds (polar, non. They are described as hydrophobic, or water fearing. Similarly, we find that polar solutes such as methanol, ethanol, and isopropanol are highly soluble in water. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is. Which Substances Cannot Dissolve In Water And Why.
From www.chegg.com
Solved Classify which compounds will dissolve in water and Which Substances Cannot Dissolve In Water And Why Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. Insoluble substance is the substance which is not dissolved in a specific dissolving media. Nonpolar molecules, such as those found in grease or oil, do not dissolve in. Make a list of substances that you know dissolve in water.. Which Substances Cannot Dissolve In Water And Why.
From www.slideserve.com
PPT Chapter 8 SOLUTIONS PowerPoint Presentation, free download ID1977818 Which Substances Cannot Dissolve In Water And Why When put into polar environments, such as water, nonpolar. Nonpolar molecules, such as those found in grease or oil, do not dissolve in. Metals, ionic compounds, molecular compounds (polar, non. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. Similarly, we find that polar solutes such as methanol,. Which Substances Cannot Dissolve In Water And Why.
From socratic.org
Substance A will not dissolve in water. What can be said about substance A? Socratic Which Substances Cannot Dissolve In Water And Why Make a list of substances that you know dissolve in water. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. Water typically dissolves most ionic compounds and polar molecules. When a solute dissolves in water, the process is referred to as hydration. Nonpolar molecules do not dissolve easily. Which Substances Cannot Dissolve In Water And Why.