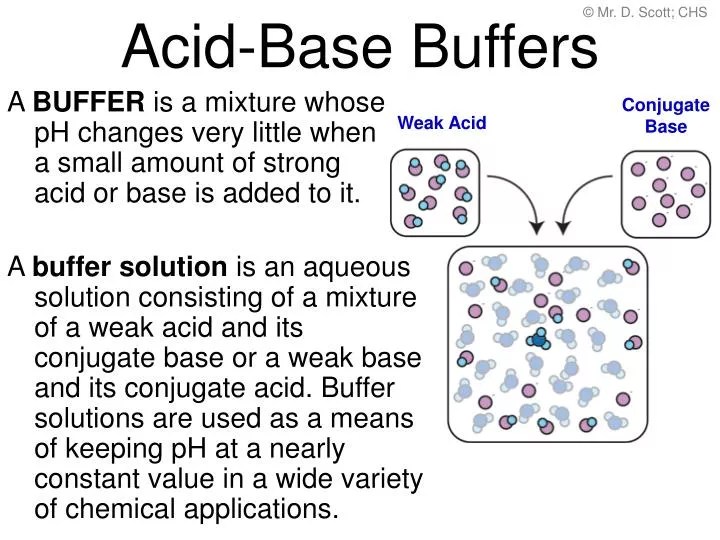
Add Acid Or Base To Buffer . A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. Calculate the ph of a buffer before and after the addition of added acid or. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is an aqueous solution that has a highly stable ph. What is a buffer solution? Conversely, when you add a base, the weak acid in the buffer neutralizes the base. Buffers function through a process of chemical equilibrium. Buffer solutions resist a change in ph when small amounts of a. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure 5.7.1). A buffering agent is a weak acid or weak base that helps maintain the ph of an aqueous solution after adding another acid or base. For example, consider a buffer made of acetic acid (ch₃cooh) and sodium acetate (ch₃coona). When you add an acid to a buffer, the conjugate base present in the buffer neutralizes it. It is able to neutralize small amounts of added acid or base, thus. If you add an acid or a base to a buffered solution, its ph will not change significantly. A solution of acetic acid.
from www.slideserve.com
A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. Buffer solutions resist a change in ph when small amounts of a. For example, consider a buffer made of acetic acid (ch₃cooh) and sodium acetate (ch₃coona). If you add an acid or a base to a buffered solution, its ph will not change significantly. A buffering agent is a weak acid or weak base that helps maintain the ph of an aqueous solution after adding another acid or base. A buffer is an aqueous solution that has a highly stable ph. What is a buffer solution? Calculate the ph of a buffer before and after the addition of added acid or. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure 5.7.1). It is able to neutralize small amounts of added acid or base, thus.
PPT AcidBase Buffers PowerPoint Presentation ID496487
Add Acid Or Base To Buffer A buffering agent is a weak acid or weak base that helps maintain the ph of an aqueous solution after adding another acid or base. It is able to neutralize small amounts of added acid or base, thus. If you add an acid or a base to a buffered solution, its ph will not change significantly. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. Buffer solutions resist a change in ph when small amounts of a. Conversely, when you add a base, the weak acid in the buffer neutralizes the base. A buffering agent is a weak acid or weak base that helps maintain the ph of an aqueous solution after adding another acid or base. When you add an acid to a buffer, the conjugate base present in the buffer neutralizes it. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A solution of acetic acid. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure 5.7.1). A buffer is an aqueous solution that has a highly stable ph. What is a buffer solution? Buffers function through a process of chemical equilibrium. For example, consider a buffer made of acetic acid (ch₃cooh) and sodium acetate (ch₃coona). Calculate the ph of a buffer before and after the addition of added acid or.
From fyognlubk.blob.core.windows.net
What Is A Buffer In Biology at Earl Lovelace blog Add Acid Or Base To Buffer A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. For example, consider a buffer made of acetic acid (ch₃cooh) and sodium acetate (ch₃coona). A buffer is an aqueous solution that has a highly stable ph. When you add an acid to a buffer, the conjugate base present in the buffer. Add Acid Or Base To Buffer.
From www.slideserve.com
PPT Chemistry 100 Chapter 17 PowerPoint Presentation, free download Add Acid Or Base To Buffer It is able to neutralize small amounts of added acid or base, thus. If you add an acid or a base to a buffered solution, its ph will not change significantly. When you add an acid to a buffer, the conjugate base present in the buffer neutralizes it. Calculate the ph of a buffer before and after the addition of. Add Acid Or Base To Buffer.
From www.youtube.com
Acidic and Basic buffer Identification and Preparation Tips and Tricks Add Acid Or Base To Buffer If you add an acid or a base to a buffered solution, its ph will not change significantly. Buffer solutions resist a change in ph when small amounts of a. A buffer is an aqueous solution that has a highly stable ph. What is a buffer solution? Conversely, when you add a base, the weak acid in the buffer neutralizes. Add Acid Or Base To Buffer.
From chemistrytalk.org
What is a Buffer Solution? Chemistry ChemTalk Add Acid Or Base To Buffer It is able to neutralize small amounts of added acid or base, thus. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure 5.7.1). When you add an acid to a buffer, the conjugate base present in the buffer neutralizes it. A buffer is a solution that can resist. Add Acid Or Base To Buffer.
From www.slideserve.com
PPT AcidBase Buffers PowerPoint Presentation, free download ID496487 Add Acid Or Base To Buffer For example, consider a buffer made of acetic acid (ch₃cooh) and sodium acetate (ch₃coona). A buffer is an aqueous solution that has a highly stable ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. If you add an acid or a base to a buffered solution, its ph will. Add Acid Or Base To Buffer.
From www.youtube.com
The Change in pH with the Addition of a Strong Acid to a Buffer YouTube Add Acid Or Base To Buffer A buffer is an aqueous solution that has a highly stable ph. Calculate the ph of a buffer before and after the addition of added acid or. For example, consider a buffer made of acetic acid (ch₃cooh) and sodium acetate (ch₃coona). If you add an acid or a base to a buffered solution, its ph will not change significantly. A. Add Acid Or Base To Buffer.
From dxouambuf.blob.core.windows.net
How To Use A Buffer at Albert Hill blog Add Acid Or Base To Buffer If you add an acid or a base to a buffered solution, its ph will not change significantly. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure 5.7.1). A buffer is an aqueous solution that has a highly stable ph. Conversely, when you add a base, the weak. Add Acid Or Base To Buffer.
From www.slideserve.com
PPT Chapter 2 PowerPoint Presentation, free download ID3141916 Add Acid Or Base To Buffer Buffers function through a process of chemical equilibrium. Conversely, when you add a base, the weak acid in the buffer neutralizes the base. Buffer solutions resist a change in ph when small amounts of a. It is able to neutralize small amounts of added acid or base, thus. For example, consider a buffer made of acetic acid (ch₃cooh) and sodium. Add Acid Or Base To Buffer.
From www.slideserve.com
PPT Unit 18 AcidBase Equilibria Buffers & Hydrolysis PowerPoint Add Acid Or Base To Buffer Buffers function through a process of chemical equilibrium. If you add an acid or a base to a buffered solution, its ph will not change significantly. Buffer solutions resist a change in ph when small amounts of a. A solution of acetic acid. A buffer is an aqueous solution that has a highly stable ph. A buffering agent is a. Add Acid Or Base To Buffer.
From www.pinterest.es
Understanding Buffer Solution Chemistry A Complete Guide Add Acid Or Base To Buffer What is a buffer solution? A solution of acetic acid. Conversely, when you add a base, the weak acid in the buffer neutralizes the base. It is able to neutralize small amounts of added acid or base, thus. A buffering agent is a weak acid or weak base that helps maintain the ph of an aqueous solution after adding another. Add Acid Or Base To Buffer.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Add Acid Or Base To Buffer When you add an acid to a buffer, the conjugate base present in the buffer neutralizes it. A buffering agent is a weak acid or weak base that helps maintain the ph of an aqueous solution after adding another acid or base. A buffer is a solution that can resist ph change upon the addition of an acidic or basic. Add Acid Or Base To Buffer.
From www.youtube.com
Adding Acid to a Buffer Calculating the pH using Henderson Add Acid Or Base To Buffer A buffer is an aqueous solution that has a highly stable ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffers function through a process of chemical equilibrium. Calculate the ph of a buffer before and after the addition of added acid or. A solution of acetic acid. Conversely,. Add Acid Or Base To Buffer.
From www.slideserve.com
PPT Chapter 10 Acids and Bases PowerPoint Presentation, free download Add Acid Or Base To Buffer A buffering agent is a weak acid or weak base that helps maintain the ph of an aqueous solution after adding another acid or base. It is able to neutralize small amounts of added acid or base, thus. What is a buffer solution? For example, consider a buffer made of acetic acid (ch₃cooh) and sodium acetate (ch₃coona). A buffer solution. Add Acid Or Base To Buffer.
From www.hopaxfc.com
How can Biological Buffer maintain the pH value? Blog Hopax Fine Add Acid Or Base To Buffer It is able to neutralize small amounts of added acid or base, thus. When you add an acid to a buffer, the conjugate base present in the buffer neutralizes it. Buffer solutions resist a change in ph when small amounts of a. A buffer is an aqueous solution that has a highly stable ph. Conversely, when you add a base,. Add Acid Or Base To Buffer.
From slideplayer.com
SCH4U Acids and Bases BUFFER SOLUTIONS. ppt download Add Acid Or Base To Buffer If you add an acid or a base to a buffered solution, its ph will not change significantly. A solution of acetic acid. A buffer is an aqueous solution that has a highly stable ph. What is a buffer solution? Conversely, when you add a base, the weak acid in the buffer neutralizes the base. A buffer is a solution. Add Acid Or Base To Buffer.
From www.slideserve.com
PPT Chapter 17 PowerPoint Presentation ID307398 Add Acid Or Base To Buffer Buffer solutions resist a change in ph when small amounts of a. It is able to neutralize small amounts of added acid or base, thus. When you add an acid to a buffer, the conjugate base present in the buffer neutralizes it. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base. Add Acid Or Base To Buffer.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent Add Acid Or Base To Buffer Calculate the ph of a buffer before and after the addition of added acid or. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. A buffering agent is a weak acid or weak base that helps maintain the ph of an aqueous solution after adding another. Add Acid Or Base To Buffer.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID4587814 Add Acid Or Base To Buffer For example, consider a buffer made of acetic acid (ch₃cooh) and sodium acetate (ch₃coona). Calculate the ph of a buffer before and after the addition of added acid or. If you add an acid or a base to a buffered solution, its ph will not change significantly. A solution of acetic acid. Buffer solutions resist a change in ph when. Add Acid Or Base To Buffer.
From courses.lumenlearning.com
Buffers Chemistry Add Acid Or Base To Buffer For example, consider a buffer made of acetic acid (ch₃cooh) and sodium acetate (ch₃coona). Buffer solutions resist a change in ph when small amounts of a. When you add an acid to a buffer, the conjugate base present in the buffer neutralizes it. A buffer solution is one which resists changes in ph when small quantities of an acid or. Add Acid Or Base To Buffer.
From www.slideserve.com
PPT Blood Gases, pH and Buffer system PowerPoint Presentation ID257615 Add Acid Or Base To Buffer A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure 5.7.1). It is able to neutralize small amounts of added acid or base, thus. A buffering agent is a weak. Add Acid Or Base To Buffer.
From classnotes.org.in
Buffer solution and Buffer Action Chemistry, Class 11, Ionic Equilibrium Add Acid Or Base To Buffer If you add an acid or a base to a buffered solution, its ph will not change significantly. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure 5.7.1). Conversely, when you add a base, the weak acid in the buffer neutralizes the base. A buffer is an aqueous. Add Acid Or Base To Buffer.
From www.youtube.com
Introduction to Buffer System Regulation of pH Acid Base Balance Add Acid Or Base To Buffer Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure 5.7.1). Buffers function through a process of chemical equilibrium. A buffering agent is a weak acid or weak base that helps maintain the ph of an aqueous solution after adding another acid or base. If you add an acid. Add Acid Or Base To Buffer.
From www.slideserve.com
PPT Chapter 17 Additional Aspects of Aqueous Equilibria PowerPoint Add Acid Or Base To Buffer Conversely, when you add a base, the weak acid in the buffer neutralizes the base. When you add an acid to a buffer, the conjugate base present in the buffer neutralizes it. If you add an acid or a base to a buffered solution, its ph will not change significantly. Buffer solutions resist a change in ph when small amounts. Add Acid Or Base To Buffer.
From labpedia.net
Acidbase Balance Part 3 Respiratory Acidosis and Respiratory Add Acid Or Base To Buffer A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. Buffers function through a process of chemical equilibrium. A buffering agent is a weak acid or weak base that helps maintain the ph of an aqueous solution after adding another acid or base. It is able to. Add Acid Or Base To Buffer.
From studylib.net
AcidBase Balance and Buffers Add Acid Or Base To Buffer A buffer is an aqueous solution that has a highly stable ph. A solution of acetic acid. Conversely, when you add a base, the weak acid in the buffer neutralizes the base. For example, consider a buffer made of acetic acid (ch₃cooh) and sodium acetate (ch₃coona). Buffer solutions resist a change in ph when small amounts of a. A buffer. Add Acid Or Base To Buffer.
From www.slideserve.com
PPT AcidBase Buffers PowerPoint Presentation ID496487 Add Acid Or Base To Buffer Buffers function through a process of chemical equilibrium. A buffer is an aqueous solution that has a highly stable ph. Buffer solutions resist a change in ph when small amounts of a. What is a buffer solution? A solution of acetic acid. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base. Add Acid Or Base To Buffer.
From www.slideserve.com
PPT Chapter 10 Acids and Bases PowerPoint Presentation, free download Add Acid Or Base To Buffer A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. A buffer is an aqueous solution that has a highly stable ph. What is a buffer solution? A buffering agent is a weak acid or weak base that helps maintain the ph of an aqueous solution after. Add Acid Or Base To Buffer.
From www.youtube.com
Buffer from weak acid and strong base YouTube Add Acid Or Base To Buffer For example, consider a buffer made of acetic acid (ch₃cooh) and sodium acetate (ch₃coona). It is able to neutralize small amounts of added acid or base, thus. A buffer is an aqueous solution that has a highly stable ph. When you add an acid to a buffer, the conjugate base present in the buffer neutralizes it. Buffer solutions resist a. Add Acid Or Base To Buffer.
From www.youtube.com
pH of a buffer after strong acid addition YouTube Add Acid Or Base To Buffer Buffers function through a process of chemical equilibrium. Calculate the ph of a buffer before and after the addition of added acid or. For example, consider a buffer made of acetic acid (ch₃cooh) and sodium acetate (ch₃coona). A solution of acetic acid. If you add an acid or a base to a buffered solution, its ph will not change significantly.. Add Acid Or Base To Buffer.
From lifeboat.com
Acids, Bases, PH, Buffers Add Acid Or Base To Buffer A solution of acetic acid. For example, consider a buffer made of acetic acid (ch₃cooh) and sodium acetate (ch₃coona). Buffers function through a process of chemical equilibrium. What is a buffer solution? Buffer solutions resist a change in ph when small amounts of a. A buffer solution is one which resists changes in ph when small quantities of an acid. Add Acid Or Base To Buffer.
From www.slideserve.com
PPT Buffering Capacity Addition of STRONG Acids or Bases PowerPoint Add Acid Or Base To Buffer A buffer is an aqueous solution that has a highly stable ph. If you add an acid or a base to a buffered solution, its ph will not change significantly. What is a buffer solution? Conversely, when you add a base, the weak acid in the buffer neutralizes the base. It is able to neutralize small amounts of added acid. Add Acid Or Base To Buffer.
From mavink.com
Acids Bases And Buffers Add Acid Or Base To Buffer If you add an acid or a base to a buffered solution, its ph will not change significantly. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure 5.7.1). It. Add Acid Or Base To Buffer.
From www.slideserve.com
PPT Unit 18 AcidBase Equilibria Buffers & Hydrolysis PowerPoint Add Acid Or Base To Buffer What is a buffer solution? Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure 5.7.1). Buffers function through a process of chemical equilibrium. A buffering agent is a weak acid or weak base that helps maintain the ph of an aqueous solution after adding another acid or base.. Add Acid Or Base To Buffer.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Add Acid Or Base To Buffer Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure 5.7.1). Calculate the ph of a buffer before and after the addition of added acid or. A buffer is an aqueous solution that has a highly stable ph. A buffer is a solution that can resist ph change upon. Add Acid Or Base To Buffer.
From www.slideserve.com
PPT Chemistry 100 Chapter 17 PowerPoint Presentation, free download Add Acid Or Base To Buffer It is able to neutralize small amounts of added acid or base, thus. A buffering agent is a weak acid or weak base that helps maintain the ph of an aqueous solution after adding another acid or base. What is a buffer solution? A buffer solution is one which resists changes in ph when small quantities of an acid or. Add Acid Or Base To Buffer.