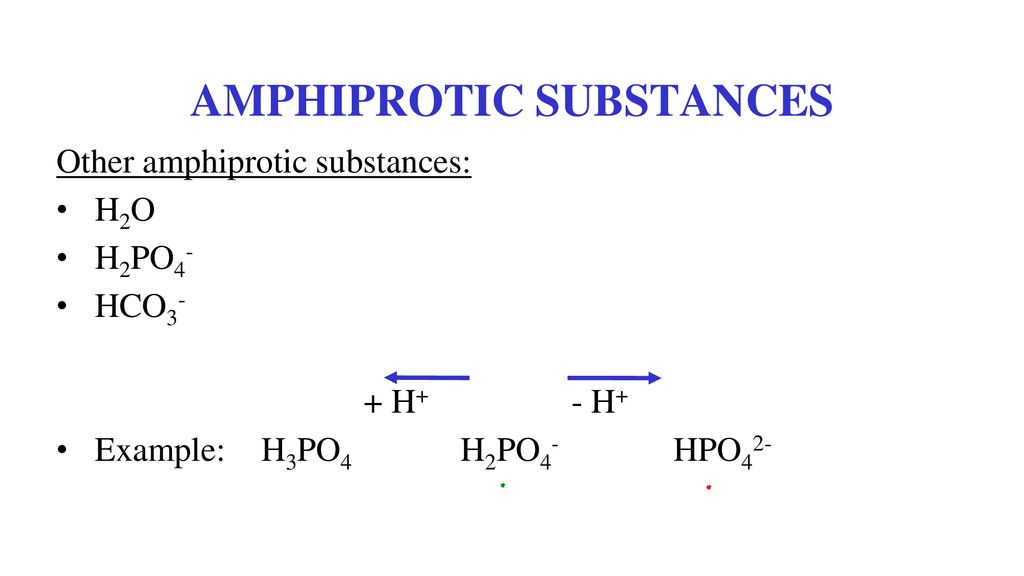
Example Of Amphiprotic Solvent . Ai generated definition based on:. Amphiprotic describes a substance that can both accept and donate a proton or h +. It is an example of a type of amphoteric molecule. A common example is the hydrogen carbonate ion, which can act as a base: An example of this is aluminium oxide which reacts with both hydrochloric acid and sodium hydroxide: A l 2 o 3 (s) + 6 hcl (aq) → 2 alcl 3 (aq) + 3h 2 o (l) a l 2 o 3 (s) + 2 naoh (aq) + 3 h. It can liberate its proton to form an aqueous solution of positively charged. What is an example of an amphiprotic substance? The most important amphiprotic species is water itself. An amphiprotic solvent is a type of solvent that can act as both an acid and a base in a chemical reaction. Molecules or ions which can either donate or accept a proton, depending on their circumstances, are called amphiprotic species. The most common example of an amphiprotic substance is water. When an acid donates a proton to water, the water molecule is a proton acceptor, and hence a base. Water can accept a proton and act as a base, but it can also donate a proton to. An amphiprotic molecule has characteristics of both and acid and a base and can act as either.
from slideplayer.com
The most important amphiprotic species is water itself. A l 2 o 3 (s) + 6 hcl (aq) → 2 alcl 3 (aq) + 3h 2 o (l) a l 2 o 3 (s) + 2 naoh (aq) + 3 h. Molecules or ions which can either donate or accept a proton, depending on their circumstances, are called amphiprotic species. Water can accept a proton and act as a base, but it can also donate a proton to. An amphiprotic molecule has characteristics of both and acid and a base and can act as either. It can liberate its proton to form an aqueous solution of positively charged. When an acid donates a proton to water, the water molecule is a proton acceptor, and hence a base. A common example is the hydrogen carbonate ion, which can act as a base: What is an example of an amphiprotic substance? An amphiprotic solvent is a type of solvent that can act as both an acid and a base in a chemical reaction.
Acids and Bases Lesson 2 Strong and Weak Bases. ppt download
Example Of Amphiprotic Solvent The most important amphiprotic species is water itself. An example of this is aluminium oxide which reacts with both hydrochloric acid and sodium hydroxide: The most important amphiprotic species is water itself. It is an example of a type of amphoteric molecule. What is an example of an amphiprotic substance? It can liberate its proton to form an aqueous solution of positively charged. An amphiprotic molecule has characteristics of both and acid and a base and can act as either. Water can accept a proton and act as a base, but it can also donate a proton to. A l 2 o 3 (s) + 6 hcl (aq) → 2 alcl 3 (aq) + 3h 2 o (l) a l 2 o 3 (s) + 2 naoh (aq) + 3 h. A common example is the hydrogen carbonate ion, which can act as a base: Molecules or ions which can either donate or accept a proton, depending on their circumstances, are called amphiprotic species. An amphiprotic solvent is a type of solvent that can act as both an acid and a base in a chemical reaction. Ai generated definition based on:. Amphiprotic describes a substance that can both accept and donate a proton or h +. The most common example of an amphiprotic substance is water. When an acid donates a proton to water, the water molecule is a proton acceptor, and hence a base.
From www.slideserve.com
PPT Chapter 9 Aqueous Solutions and Chemical Equilibria PowerPoint Example Of Amphiprotic Solvent The most common example of an amphiprotic substance is water. Amphiprotic describes a substance that can both accept and donate a proton or h +. Water can accept a proton and act as a base, but it can also donate a proton to. Ai generated definition based on:. It is an example of a type of amphoteric molecule. What is. Example Of Amphiprotic Solvent.
From chemawareness.blogspot.com
Chem Awareness Classification of Solvent`s Types of Solvents Examples, Example Of Amphiprotic Solvent A common example is the hydrogen carbonate ion, which can act as a base: Ai generated definition based on:. It can liberate its proton to form an aqueous solution of positively charged. A l 2 o 3 (s) + 6 hcl (aq) → 2 alcl 3 (aq) + 3h 2 o (l) a l 2 o 3 (s) + 2. Example Of Amphiprotic Solvent.
From www.slideserve.com
PPT Chapter 3 Chemical Reactions PowerPoint Presentation, free Example Of Amphiprotic Solvent An amphiprotic solvent is a type of solvent that can act as both an acid and a base in a chemical reaction. It can liberate its proton to form an aqueous solution of positively charged. Water can accept a proton and act as a base, but it can also donate a proton to. Molecules or ions which can either donate. Example Of Amphiprotic Solvent.
From www.youtube.com
NON AQUEOUS TITRATION/ TYPES OF SOLVENT USED/ APROTIC/AMPHIPROTIC Example Of Amphiprotic Solvent When an acid donates a proton to water, the water molecule is a proton acceptor, and hence a base. A l 2 o 3 (s) + 6 hcl (aq) → 2 alcl 3 (aq) + 3h 2 o (l) a l 2 o 3 (s) + 2 naoh (aq) + 3 h. The most important amphiprotic species is water itself.. Example Of Amphiprotic Solvent.
From slideplayer.com
Acids and Bases Lesson 2 Strong and Weak Bases. ppt download Example Of Amphiprotic Solvent It can liberate its proton to form an aqueous solution of positively charged. What is an example of an amphiprotic substance? Water can accept a proton and act as a base, but it can also donate a proton to. It is an example of a type of amphoteric molecule. When an acid donates a proton to water, the water molecule. Example Of Amphiprotic Solvent.
From slideplayer.com
Chemistry AS C3.6 Aqueous Systems. ppt download Example Of Amphiprotic Solvent An amphiprotic solvent is a type of solvent that can act as both an acid and a base in a chemical reaction. It can liberate its proton to form an aqueous solution of positively charged. Molecules or ions which can either donate or accept a proton, depending on their circumstances, are called amphiprotic species. When an acid donates a proton. Example Of Amphiprotic Solvent.
From www.expii.com
Amphiprotic vs. Amphoteric — Comparison & Examples Expii Example Of Amphiprotic Solvent The most common example of an amphiprotic substance is water. Water can accept a proton and act as a base, but it can also donate a proton to. An example of this is aluminium oxide which reacts with both hydrochloric acid and sodium hydroxide: What is an example of an amphiprotic substance? A l 2 o 3 (s) + 6. Example Of Amphiprotic Solvent.
From www.slideserve.com
PPT NON AQUEOUS SOLVENTS PowerPoint Presentation, free download ID Example Of Amphiprotic Solvent Amphiprotic describes a substance that can both accept and donate a proton or h +. The most common example of an amphiprotic substance is water. Molecules or ions which can either donate or accept a proton, depending on their circumstances, are called amphiprotic species. When an acid donates a proton to water, the water molecule is a proton acceptor, and. Example Of Amphiprotic Solvent.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID6416259 Example Of Amphiprotic Solvent It can liberate its proton to form an aqueous solution of positively charged. An amphiprotic solvent is a type of solvent that can act as both an acid and a base in a chemical reaction. It is an example of a type of amphoteric molecule. The most important amphiprotic species is water itself. A l 2 o 3 (s) +. Example Of Amphiprotic Solvent.
From www.slideserve.com
PPT Chapter 4 PowerPoint Presentation, free download ID269665 Example Of Amphiprotic Solvent A l 2 o 3 (s) + 6 hcl (aq) → 2 alcl 3 (aq) + 3h 2 o (l) a l 2 o 3 (s) + 2 naoh (aq) + 3 h. It can liberate its proton to form an aqueous solution of positively charged. It is an example of a type of amphoteric molecule. An amphiprotic molecule has. Example Of Amphiprotic Solvent.
From www.chegg.com
Solved State which of the following species are amphiprotic Example Of Amphiprotic Solvent The most common example of an amphiprotic substance is water. It can liberate its proton to form an aqueous solution of positively charged. Water can accept a proton and act as a base, but it can also donate a proton to. It is an example of a type of amphoteric molecule. An amphiprotic solvent is a type of solvent that. Example Of Amphiprotic Solvent.
From www.slideserve.com
PPT Equilibrium Part 2 Chemistry 2 PowerPoint Presentation, free Example Of Amphiprotic Solvent A common example is the hydrogen carbonate ion, which can act as a base: Molecules or ions which can either donate or accept a proton, depending on their circumstances, are called amphiprotic species. Amphiprotic describes a substance that can both accept and donate a proton or h +. An example of this is aluminium oxide which reacts with both hydrochloric. Example Of Amphiprotic Solvent.
From slideplayer.com
OVERVIEW THE CHEMICAL COMPOSITION OF AQUEOUS SOLUTIONS ppt download Example Of Amphiprotic Solvent A common example is the hydrogen carbonate ion, which can act as a base: The most common example of an amphiprotic substance is water. Ai generated definition based on:. Amphiprotic describes a substance that can both accept and donate a proton or h +. An example of this is aluminium oxide which reacts with both hydrochloric acid and sodium hydroxide:. Example Of Amphiprotic Solvent.
From www.chegg.com
Solved 4/25 Next An example of an amphiprotic solvent is O Example Of Amphiprotic Solvent Molecules or ions which can either donate or accept a proton, depending on their circumstances, are called amphiprotic species. Water can accept a proton and act as a base, but it can also donate a proton to. Ai generated definition based on:. It is an example of a type of amphoteric molecule. What is an example of an amphiprotic substance?. Example Of Amphiprotic Solvent.
From www.slideserve.com
PPT Historical Development of Acid/Base Theories PowerPoint Example Of Amphiprotic Solvent Water can accept a proton and act as a base, but it can also donate a proton to. Ai generated definition based on:. It is an example of a type of amphoteric molecule. Molecules or ions which can either donate or accept a proton, depending on their circumstances, are called amphiprotic species. An example of this is aluminium oxide which. Example Of Amphiprotic Solvent.
From www.slideserve.com
PPT NON AQUEOUS SOLVENTS PowerPoint Presentation, free download ID Example Of Amphiprotic Solvent A common example is the hydrogen carbonate ion, which can act as a base: It can liberate its proton to form an aqueous solution of positively charged. An example of this is aluminium oxide which reacts with both hydrochloric acid and sodium hydroxide: When an acid donates a proton to water, the water molecule is a proton acceptor, and hence. Example Of Amphiprotic Solvent.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID546616 Example Of Amphiprotic Solvent An amphiprotic molecule has characteristics of both and acid and a base and can act as either. When an acid donates a proton to water, the water molecule is a proton acceptor, and hence a base. It can liberate its proton to form an aqueous solution of positively charged. Molecules or ions which can either donate or accept a proton,. Example Of Amphiprotic Solvent.
From slideplayer.com
Analytical Chemistry. Analytical Chemistry References 1Fundamental Example Of Amphiprotic Solvent Amphiprotic describes a substance that can both accept and donate a proton or h +. The most important amphiprotic species is water itself. When an acid donates a proton to water, the water molecule is a proton acceptor, and hence a base. What is an example of an amphiprotic substance? Water can accept a proton and act as a base,. Example Of Amphiprotic Solvent.
From www.numerade.com
SOLVEDWhat is an amphiprotic species? Name one and write balanced Example Of Amphiprotic Solvent An amphiprotic solvent is a type of solvent that can act as both an acid and a base in a chemical reaction. The most common example of an amphiprotic substance is water. It is an example of a type of amphoteric molecule. An example of this is aluminium oxide which reacts with both hydrochloric acid and sodium hydroxide: A common. Example Of Amphiprotic Solvent.
From www.youtube.com
types of solvent YouTube Example Of Amphiprotic Solvent The most important amphiprotic species is water itself. It is an example of a type of amphoteric molecule. When an acid donates a proton to water, the water molecule is a proton acceptor, and hence a base. The most common example of an amphiprotic substance is water. It can liberate its proton to form an aqueous solution of positively charged.. Example Of Amphiprotic Solvent.
From www.chegg.com
Solved An example of an amphiprotic solvent is O Water O Example Of Amphiprotic Solvent It can liberate its proton to form an aqueous solution of positively charged. A l 2 o 3 (s) + 6 hcl (aq) → 2 alcl 3 (aq) + 3h 2 o (l) a l 2 o 3 (s) + 2 naoh (aq) + 3 h. An amphiprotic molecule has characteristics of both and acid and a base and can. Example Of Amphiprotic Solvent.
From www.youtube.com
Amphiprotic Substances // HSC Chemistry YouTube Example Of Amphiprotic Solvent What is an example of an amphiprotic substance? Water can accept a proton and act as a base, but it can also donate a proton to. Ai generated definition based on:. It is an example of a type of amphoteric molecule. Amphiprotic describes a substance that can both accept and donate a proton or h +. An amphiprotic molecule has. Example Of Amphiprotic Solvent.
From www.thoughtco.com
Amphiprotic Definition in Chemistry Example Of Amphiprotic Solvent Ai generated definition based on:. Water can accept a proton and act as a base, but it can also donate a proton to. Amphiprotic describes a substance that can both accept and donate a proton or h +. An example of this is aluminium oxide which reacts with both hydrochloric acid and sodium hydroxide: It is an example of a. Example Of Amphiprotic Solvent.
From www.chegg.com
Solved 14/25 Next An example of an amphiprotic solvent is O Example Of Amphiprotic Solvent The most important amphiprotic species is water itself. An example of this is aluminium oxide which reacts with both hydrochloric acid and sodium hydroxide: It can liberate its proton to form an aqueous solution of positively charged. An amphiprotic solvent is a type of solvent that can act as both an acid and a base in a chemical reaction. Ai. Example Of Amphiprotic Solvent.
From www.youtube.com
8.1 Amphiprotic species (SL) YouTube Example Of Amphiprotic Solvent It is an example of a type of amphoteric molecule. The most common example of an amphiprotic substance is water. A l 2 o 3 (s) + 6 hcl (aq) → 2 alcl 3 (aq) + 3h 2 o (l) a l 2 o 3 (s) + 2 naoh (aq) + 3 h. When an acid donates a proton to. Example Of Amphiprotic Solvent.
From www.expii.com
Amphiprotic vs. Amphoteric — Comparison & Examples Expii Example Of Amphiprotic Solvent What is an example of an amphiprotic substance? Ai generated definition based on:. A l 2 o 3 (s) + 6 hcl (aq) → 2 alcl 3 (aq) + 3h 2 o (l) a l 2 o 3 (s) + 2 naoh (aq) + 3 h. An example of this is aluminium oxide which reacts with both hydrochloric acid and. Example Of Amphiprotic Solvent.
From www.slideserve.com
PPT Topic 8 Acids and Bases PowerPoint Presentation, free download Example Of Amphiprotic Solvent It can liberate its proton to form an aqueous solution of positively charged. A common example is the hydrogen carbonate ion, which can act as a base: An amphiprotic molecule has characteristics of both and acid and a base and can act as either. A l 2 o 3 (s) + 6 hcl (aq) → 2 alcl 3 (aq) +. Example Of Amphiprotic Solvent.
From www.slideserve.com
PPT SCH4U Unit 2 EQUILIBRIUM PowerPoint Presentation, free download Example Of Amphiprotic Solvent The most important amphiprotic species is water itself. An example of this is aluminium oxide which reacts with both hydrochloric acid and sodium hydroxide: The most common example of an amphiprotic substance is water. When an acid donates a proton to water, the water molecule is a proton acceptor, and hence a base. Ai generated definition based on:. Amphiprotic describes. Example Of Amphiprotic Solvent.
From slideplayer.com
Analytical Chemistry. Analytical Chemistry References 1Fundamental Example Of Amphiprotic Solvent The most common example of an amphiprotic substance is water. The most important amphiprotic species is water itself. A common example is the hydrogen carbonate ion, which can act as a base: Molecules or ions which can either donate or accept a proton, depending on their circumstances, are called amphiprotic species. Amphiprotic describes a substance that can both accept and. Example Of Amphiprotic Solvent.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation ID3842068 Example Of Amphiprotic Solvent The most common example of an amphiprotic substance is water. When an acid donates a proton to water, the water molecule is a proton acceptor, and hence a base. Amphiprotic describes a substance that can both accept and donate a proton or h +. What is an example of an amphiprotic substance? Ai generated definition based on:. An example of. Example Of Amphiprotic Solvent.
From brainly.in
Example of amphiprotic solvent Brainly.in Example Of Amphiprotic Solvent It is an example of a type of amphoteric molecule. Molecules or ions which can either donate or accept a proton, depending on their circumstances, are called amphiprotic species. Ai generated definition based on:. An amphiprotic molecule has characteristics of both and acid and a base and can act as either. An example of this is aluminium oxide which reacts. Example Of Amphiprotic Solvent.
From www.slideserve.com
PPT SCH4U Unit 2 EQUILIBRIUM PowerPoint Presentation, free download Example Of Amphiprotic Solvent What is an example of an amphiprotic substance? A common example is the hydrogen carbonate ion, which can act as a base: Amphiprotic describes a substance that can both accept and donate a proton or h +. An amphiprotic solvent is a type of solvent that can act as both an acid and a base in a chemical reaction. Water. Example Of Amphiprotic Solvent.
From www.slideserve.com
PPT Non aqueous Titration theory and principles ppt PowerPoint Example Of Amphiprotic Solvent Amphiprotic describes a substance that can both accept and donate a proton or h +. What is an example of an amphiprotic substance? An example of this is aluminium oxide which reacts with both hydrochloric acid and sodium hydroxide: When an acid donates a proton to water, the water molecule is a proton acceptor, and hence a base. It can. Example Of Amphiprotic Solvent.
From www.slideserve.com
PPT Bases PowerPoint Presentation, free download ID9711232 Example Of Amphiprotic Solvent The most important amphiprotic species is water itself. An amphiprotic solvent is a type of solvent that can act as both an acid and a base in a chemical reaction. The most common example of an amphiprotic substance is water. Molecules or ions which can either donate or accept a proton, depending on their circumstances, are called amphiprotic species. When. Example Of Amphiprotic Solvent.
From www.youtube.com
Amphiprotic Substances Act as BOTH an Acid and a Base WATER is Example Of Amphiprotic Solvent An amphiprotic solvent is a type of solvent that can act as both an acid and a base in a chemical reaction. When an acid donates a proton to water, the water molecule is a proton acceptor, and hence a base. What is an example of an amphiprotic substance? The most common example of an amphiprotic substance is water. It. Example Of Amphiprotic Solvent.