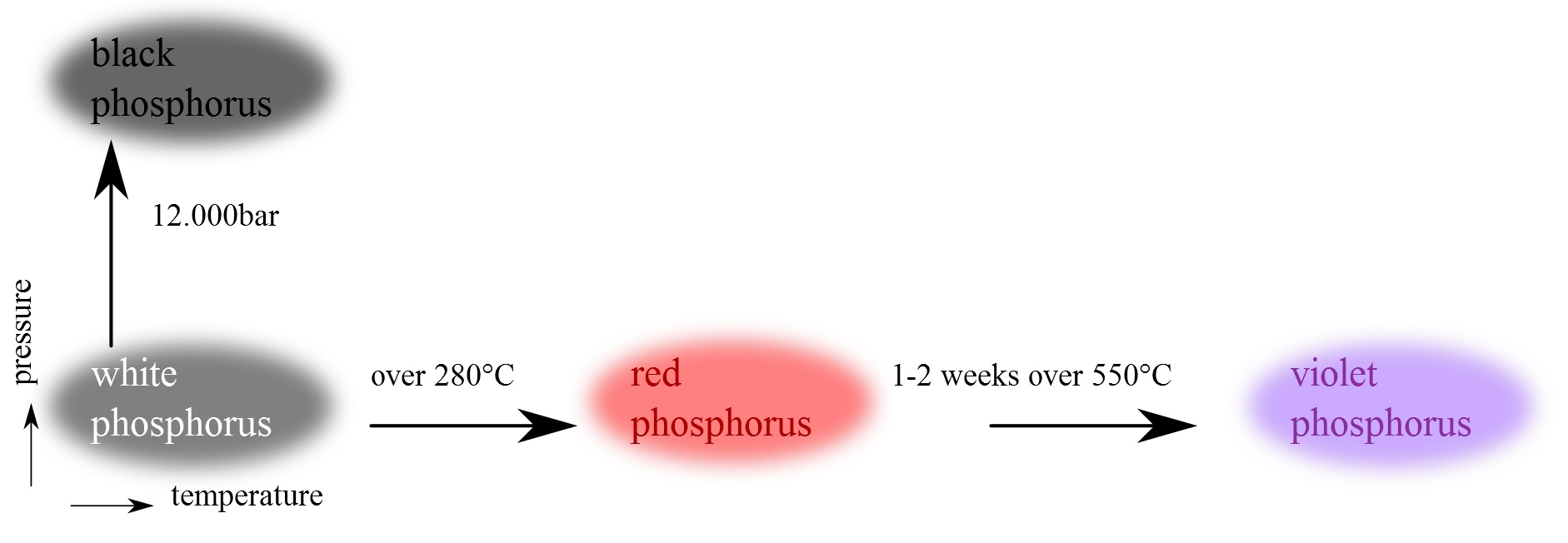
Difference Between Sulfur And Phosphorus . ionic compounds consist of positively and negatively charged ions held together by strong electrostatic forces, whereas covalent compounds generally. the oxides of phosphorus, sulfur and chlorine consist of individual molecules, simple or polymeric. i think phosphorus does have a higher ionization energy because when you look at the shells filled up, sulfur has one. as nouns the difference between phosphorus and sulfur is that phosphorus is a chemical element ( symbol p) with an atomic. phosphorus is a nonmetal, solid at room temperature, and a poor conductor of heat and electricity. Phosphorus occurs in at least 10 allotropic forms, the most. phosphorus, sulfur, chlorine and argon are simple molecular substances with only van der waals attractions. compare phosphorus and sulfur on the basis of their properties, attributes and periodic table facts.
from wwwchem.uwimona.edu.jm
i think phosphorus does have a higher ionization energy because when you look at the shells filled up, sulfur has one. compare phosphorus and sulfur on the basis of their properties, attributes and periodic table facts. phosphorus is a nonmetal, solid at room temperature, and a poor conductor of heat and electricity. as nouns the difference between phosphorus and sulfur is that phosphorus is a chemical element ( symbol p) with an atomic. the oxides of phosphorus, sulfur and chlorine consist of individual molecules, simple or polymeric. ionic compounds consist of positively and negatively charged ions held together by strong electrostatic forces, whereas covalent compounds generally. phosphorus, sulfur, chlorine and argon are simple molecular substances with only van der waals attractions. Phosphorus occurs in at least 10 allotropic forms, the most.
CHEM1902 Structure of Phosphorus, Sulfur and Iodine
Difference Between Sulfur And Phosphorus compare phosphorus and sulfur on the basis of their properties, attributes and periodic table facts. phosphorus, sulfur, chlorine and argon are simple molecular substances with only van der waals attractions. the oxides of phosphorus, sulfur and chlorine consist of individual molecules, simple or polymeric. phosphorus is a nonmetal, solid at room temperature, and a poor conductor of heat and electricity. Phosphorus occurs in at least 10 allotropic forms, the most. compare phosphorus and sulfur on the basis of their properties, attributes and periodic table facts. as nouns the difference between phosphorus and sulfur is that phosphorus is a chemical element ( symbol p) with an atomic. ionic compounds consist of positively and negatively charged ions held together by strong electrostatic forces, whereas covalent compounds generally. i think phosphorus does have a higher ionization energy because when you look at the shells filled up, sulfur has one.
From www.youtube.com
Assimilation of Sulfur and Phosphorus . Lecture no _1 YouTube Difference Between Sulfur And Phosphorus phosphorus, sulfur, chlorine and argon are simple molecular substances with only van der waals attractions. ionic compounds consist of positively and negatively charged ions held together by strong electrostatic forces, whereas covalent compounds generally. the oxides of phosphorus, sulfur and chlorine consist of individual molecules, simple or polymeric. Phosphorus occurs in at least 10 allotropic forms, the. Difference Between Sulfur And Phosphorus.
From www.slideserve.com
PPT Physiology of Bacteria PowerPoint Presentation ID5773371 Difference Between Sulfur And Phosphorus as nouns the difference between phosphorus and sulfur is that phosphorus is a chemical element ( symbol p) with an atomic. ionic compounds consist of positively and negatively charged ions held together by strong electrostatic forces, whereas covalent compounds generally. i think phosphorus does have a higher ionization energy because when you look at the shells filled. Difference Between Sulfur And Phosphorus.
From wou.edu
CH105 Chapter 10 Compounds with Sulfur, Phosphorus, and Nitrogen Difference Between Sulfur And Phosphorus compare phosphorus and sulfur on the basis of their properties, attributes and periodic table facts. as nouns the difference between phosphorus and sulfur is that phosphorus is a chemical element ( symbol p) with an atomic. the oxides of phosphorus, sulfur and chlorine consist of individual molecules, simple or polymeric. phosphorus is a nonmetal, solid at. Difference Between Sulfur And Phosphorus.
From www.differencebetween.com
Difference Between Carbon Cycle and Phosphorus Cycle Compare the Difference Between Sulfur And Phosphorus compare phosphorus and sulfur on the basis of their properties, attributes and periodic table facts. the oxides of phosphorus, sulfur and chlorine consist of individual molecules, simple or polymeric. ionic compounds consist of positively and negatively charged ions held together by strong electrostatic forces, whereas covalent compounds generally. Phosphorus occurs in at least 10 allotropic forms, the. Difference Between Sulfur And Phosphorus.
From pediaa.com
Difference Between Phosphorus and Phosphate Physical and Chemical Difference Between Sulfur And Phosphorus the oxides of phosphorus, sulfur and chlorine consist of individual molecules, simple or polymeric. phosphorus is a nonmetal, solid at room temperature, and a poor conductor of heat and electricity. ionic compounds consist of positively and negatively charged ions held together by strong electrostatic forces, whereas covalent compounds generally. Phosphorus occurs in at least 10 allotropic forms,. Difference Between Sulfur And Phosphorus.
From www.slideshare.net
The phosphorus and sulfur cycle Difference Between Sulfur And Phosphorus compare phosphorus and sulfur on the basis of their properties, attributes and periodic table facts. i think phosphorus does have a higher ionization energy because when you look at the shells filled up, sulfur has one. phosphorus is a nonmetal, solid at room temperature, and a poor conductor of heat and electricity. the oxides of phosphorus,. Difference Between Sulfur And Phosphorus.
From www.youtube.com
Phosphorus, Nitrogen and Sulphur Ylides YouTube Difference Between Sulfur And Phosphorus phosphorus is a nonmetal, solid at room temperature, and a poor conductor of heat and electricity. the oxides of phosphorus, sulfur and chlorine consist of individual molecules, simple or polymeric. ionic compounds consist of positively and negatively charged ions held together by strong electrostatic forces, whereas covalent compounds generally. Phosphorus occurs in at least 10 allotropic forms,. Difference Between Sulfur And Phosphorus.
From atarbiologyncc.weebly.com
Biogeochemical Cycling ATAR BIOLOGY Difference Between Sulfur And Phosphorus the oxides of phosphorus, sulfur and chlorine consist of individual molecules, simple or polymeric. compare phosphorus and sulfur on the basis of their properties, attributes and periodic table facts. ionic compounds consist of positively and negatively charged ions held together by strong electrostatic forces, whereas covalent compounds generally. phosphorus is a nonmetal, solid at room temperature,. Difference Between Sulfur And Phosphorus.
From www.differencebetween.com
Difference Between Carbon Cycle and Phosphorus Cycle Compare the Difference Between Sulfur And Phosphorus compare phosphorus and sulfur on the basis of their properties, attributes and periodic table facts. as nouns the difference between phosphorus and sulfur is that phosphorus is a chemical element ( symbol p) with an atomic. Phosphorus occurs in at least 10 allotropic forms, the most. phosphorus is a nonmetal, solid at room temperature, and a poor. Difference Between Sulfur And Phosphorus.
From www.slideshare.net
The phosphorus and sulfur cycle Difference Between Sulfur And Phosphorus compare phosphorus and sulfur on the basis of their properties, attributes and periodic table facts. phosphorus is a nonmetal, solid at room temperature, and a poor conductor of heat and electricity. as nouns the difference between phosphorus and sulfur is that phosphorus is a chemical element ( symbol p) with an atomic. Phosphorus occurs in at least. Difference Between Sulfur And Phosphorus.
From www.slideserve.com
PPT Chapter 8 PowerPoint Presentation, free download ID2372554 Difference Between Sulfur And Phosphorus Phosphorus occurs in at least 10 allotropic forms, the most. phosphorus, sulfur, chlorine and argon are simple molecular substances with only van der waals attractions. ionic compounds consist of positively and negatively charged ions held together by strong electrostatic forces, whereas covalent compounds generally. compare phosphorus and sulfur on the basis of their properties, attributes and periodic. Difference Between Sulfur And Phosphorus.
From www.slideshare.net
The phosphorus and sulfur cycle Difference Between Sulfur And Phosphorus i think phosphorus does have a higher ionization energy because when you look at the shells filled up, sulfur has one. ionic compounds consist of positively and negatively charged ions held together by strong electrostatic forces, whereas covalent compounds generally. phosphorus, sulfur, chlorine and argon are simple molecular substances with only van der waals attractions. the. Difference Between Sulfur And Phosphorus.
From www.differencebetween.com
Difference Between Sulfonate and Sulfate Compare the Difference Difference Between Sulfur And Phosphorus Phosphorus occurs in at least 10 allotropic forms, the most. as nouns the difference between phosphorus and sulfur is that phosphorus is a chemical element ( symbol p) with an atomic. phosphorus is a nonmetal, solid at room temperature, and a poor conductor of heat and electricity. i think phosphorus does have a higher ionization energy because. Difference Between Sulfur And Phosphorus.
From www.youtube.com
Phosphorus vs Phosphate YouTube Difference Between Sulfur And Phosphorus i think phosphorus does have a higher ionization energy because when you look at the shells filled up, sulfur has one. phosphorus is a nonmetal, solid at room temperature, and a poor conductor of heat and electricity. phosphorus, sulfur, chlorine and argon are simple molecular substances with only van der waals attractions. the oxides of phosphorus,. Difference Between Sulfur And Phosphorus.
From www.chem.tamu.edu
Phosphorus and Phosphoric Acid Difference Between Sulfur And Phosphorus i think phosphorus does have a higher ionization energy because when you look at the shells filled up, sulfur has one. Phosphorus occurs in at least 10 allotropic forms, the most. phosphorus, sulfur, chlorine and argon are simple molecular substances with only van der waals attractions. the oxides of phosphorus, sulfur and chlorine consist of individual molecules,. Difference Between Sulfur And Phosphorus.
From www.dreamstime.com
Sulfur,Phosphorus on the Periodic Table of the Elements on White Difference Between Sulfur And Phosphorus Phosphorus occurs in at least 10 allotropic forms, the most. ionic compounds consist of positively and negatively charged ions held together by strong electrostatic forces, whereas covalent compounds generally. phosphorus, sulfur, chlorine and argon are simple molecular substances with only van der waals attractions. as nouns the difference between phosphorus and sulfur is that phosphorus is a. Difference Between Sulfur And Phosphorus.
From www.numerade.com
SOLVEDUse Figure 7.4 to calculate the difference in electronegativity Difference Between Sulfur And Phosphorus phosphorus is a nonmetal, solid at room temperature, and a poor conductor of heat and electricity. i think phosphorus does have a higher ionization energy because when you look at the shells filled up, sulfur has one. the oxides of phosphorus, sulfur and chlorine consist of individual molecules, simple or polymeric. Phosphorus occurs in at least 10. Difference Between Sulfur And Phosphorus.
From wwwchem.uwimona.edu.jm
CHEM1902 Structure of Phosphorus, Sulfur and Iodine Difference Between Sulfur And Phosphorus ionic compounds consist of positively and negatively charged ions held together by strong electrostatic forces, whereas covalent compounds generally. compare phosphorus and sulfur on the basis of their properties, attributes and periodic table facts. i think phosphorus does have a higher ionization energy because when you look at the shells filled up, sulfur has one. as. Difference Between Sulfur And Phosphorus.
From www.youtube.com
Seminar 6 The Phosphorus & Sulfur Cycle Part 2 YouTube Difference Between Sulfur And Phosphorus compare phosphorus and sulfur on the basis of their properties, attributes and periodic table facts. phosphorus, sulfur, chlorine and argon are simple molecular substances with only van der waals attractions. phosphorus is a nonmetal, solid at room temperature, and a poor conductor of heat and electricity. Phosphorus occurs in at least 10 allotropic forms, the most. . Difference Between Sulfur And Phosphorus.
From www.sciencephoto.com
The elements phosphorus and sulphur Stock Image A150/0211 Science Difference Between Sulfur And Phosphorus i think phosphorus does have a higher ionization energy because when you look at the shells filled up, sulfur has one. compare phosphorus and sulfur on the basis of their properties, attributes and periodic table facts. the oxides of phosphorus, sulfur and chlorine consist of individual molecules, simple or polymeric. Phosphorus occurs in at least 10 allotropic. Difference Between Sulfur And Phosphorus.
From www.youtube.com
Nitrogen, Phosphorus and Sulfur cycles YouTube Difference Between Sulfur And Phosphorus phosphorus is a nonmetal, solid at room temperature, and a poor conductor of heat and electricity. as nouns the difference between phosphorus and sulfur is that phosphorus is a chemical element ( symbol p) with an atomic. ionic compounds consist of positively and negatively charged ions held together by strong electrostatic forces, whereas covalent compounds generally. . Difference Between Sulfur And Phosphorus.
From pediaa.com
Difference Between Sulphuric Acid and Sulphurous Acid Definition Difference Between Sulfur And Phosphorus i think phosphorus does have a higher ionization energy because when you look at the shells filled up, sulfur has one. Phosphorus occurs in at least 10 allotropic forms, the most. phosphorus is a nonmetal, solid at room temperature, and a poor conductor of heat and electricity. phosphorus, sulfur, chlorine and argon are simple molecular substances with. Difference Between Sulfur And Phosphorus.
From www.differencebetween.net
Difference Between Phosphorus and Phosphate Difference Between Difference Between Sulfur And Phosphorus phosphorus, sulfur, chlorine and argon are simple molecular substances with only van der waals attractions. i think phosphorus does have a higher ionization energy because when you look at the shells filled up, sulfur has one. Phosphorus occurs in at least 10 allotropic forms, the most. ionic compounds consist of positively and negatively charged ions held together. Difference Between Sulfur And Phosphorus.
From www.youtube.com
The Phosphorus & Sulphur Cycle IBornot2B YouTube Difference Between Sulfur And Phosphorus phosphorus is a nonmetal, solid at room temperature, and a poor conductor of heat and electricity. ionic compounds consist of positively and negatively charged ions held together by strong electrostatic forces, whereas covalent compounds generally. the oxides of phosphorus, sulfur and chlorine consist of individual molecules, simple or polymeric. Phosphorus occurs in at least 10 allotropic forms,. Difference Between Sulfur And Phosphorus.
From www.differencebetween.com
Difference Between Ammonium Sulfate and Sodium Sulphate Compare the Difference Between Sulfur And Phosphorus as nouns the difference between phosphorus and sulfur is that phosphorus is a chemical element ( symbol p) with an atomic. the oxides of phosphorus, sulfur and chlorine consist of individual molecules, simple or polymeric. phosphorus, sulfur, chlorine and argon are simple molecular substances with only van der waals attractions. Phosphorus occurs in at least 10 allotropic. Difference Between Sulfur And Phosphorus.
From www.slideserve.com
PPT Chemistry Nomenclature Acid Nomenclature PowerPoint Difference Between Sulfur And Phosphorus compare phosphorus and sulfur on the basis of their properties, attributes and periodic table facts. as nouns the difference between phosphorus and sulfur is that phosphorus is a chemical element ( symbol p) with an atomic. ionic compounds consist of positively and negatively charged ions held together by strong electrostatic forces, whereas covalent compounds generally. phosphorus,. Difference Between Sulfur And Phosphorus.
From www.slideserve.com
PPT Bioremediation PowerPoint Presentation, free download ID1427954 Difference Between Sulfur And Phosphorus as nouns the difference between phosphorus and sulfur is that phosphorus is a chemical element ( symbol p) with an atomic. phosphorus is a nonmetal, solid at room temperature, and a poor conductor of heat and electricity. phosphorus, sulfur, chlorine and argon are simple molecular substances with only van der waals attractions. Phosphorus occurs in at least. Difference Between Sulfur And Phosphorus.
From www.buddingforensicexpert.in
Difference between Sulfur and Sulphur Budding Forensic Expert Difference Between Sulfur And Phosphorus compare phosphorus and sulfur on the basis of their properties, attributes and periodic table facts. phosphorus, sulfur, chlorine and argon are simple molecular substances with only van der waals attractions. phosphorus is a nonmetal, solid at room temperature, and a poor conductor of heat and electricity. as nouns the difference between phosphorus and sulfur is that. Difference Between Sulfur And Phosphorus.
From www.slideserve.com
PPT Allotropes Awww Yeahh PowerPoint Presentation, free download ID Difference Between Sulfur And Phosphorus ionic compounds consist of positively and negatively charged ions held together by strong electrostatic forces, whereas covalent compounds generally. Phosphorus occurs in at least 10 allotropic forms, the most. i think phosphorus does have a higher ionization energy because when you look at the shells filled up, sulfur has one. phosphorus is a nonmetal, solid at room. Difference Between Sulfur And Phosphorus.
From dokumen.tips
(PPT) Phosphorus and Sulfur Cycles. Facts about the Phosphorus cycle Difference Between Sulfur And Phosphorus Phosphorus occurs in at least 10 allotropic forms, the most. the oxides of phosphorus, sulfur and chlorine consist of individual molecules, simple or polymeric. phosphorus, sulfur, chlorine and argon are simple molecular substances with only van der waals attractions. i think phosphorus does have a higher ionization energy because when you look at the shells filled up,. Difference Between Sulfur And Phosphorus.
From wou.edu
CH105 Chapter 10 Compounds with Sulfur, Phosphorus, and Nitrogen Difference Between Sulfur And Phosphorus phosphorus, sulfur, chlorine and argon are simple molecular substances with only van der waals attractions. ionic compounds consist of positively and negatively charged ions held together by strong electrostatic forces, whereas covalent compounds generally. compare phosphorus and sulfur on the basis of their properties, attributes and periodic table facts. as nouns the difference between phosphorus and. Difference Between Sulfur And Phosphorus.
From pediaa.com
Difference Between Sulfur and Sulfur Dioxide Definition, Physical and Difference Between Sulfur And Phosphorus phosphorus, sulfur, chlorine and argon are simple molecular substances with only van der waals attractions. ionic compounds consist of positively and negatively charged ions held together by strong electrostatic forces, whereas covalent compounds generally. i think phosphorus does have a higher ionization energy because when you look at the shells filled up, sulfur has one. compare. Difference Between Sulfur And Phosphorus.
From pediaa.com
Difference Between Sulfur and Sulfaten Properties, Allotropes Difference Between Sulfur And Phosphorus i think phosphorus does have a higher ionization energy because when you look at the shells filled up, sulfur has one. ionic compounds consist of positively and negatively charged ions held together by strong electrostatic forces, whereas covalent compounds generally. compare phosphorus and sulfur on the basis of their properties, attributes and periodic table facts. phosphorus,. Difference Between Sulfur And Phosphorus.
From www.youtube.com
Unit 3 Pt 3 Phosphorus, Sulfur, Water Cycles AP Environmental YouTube Difference Between Sulfur And Phosphorus i think phosphorus does have a higher ionization energy because when you look at the shells filled up, sulfur has one. phosphorus is a nonmetal, solid at room temperature, and a poor conductor of heat and electricity. as nouns the difference between phosphorus and sulfur is that phosphorus is a chemical element ( symbol p) with an. Difference Between Sulfur And Phosphorus.
From www.onlyiasexam.com
Principle of Biogeochemical cycles Principle of ecology UPSC Difference Between Sulfur And Phosphorus compare phosphorus and sulfur on the basis of their properties, attributes and periodic table facts. phosphorus, sulfur, chlorine and argon are simple molecular substances with only van der waals attractions. i think phosphorus does have a higher ionization energy because when you look at the shells filled up, sulfur has one. as nouns the difference between. Difference Between Sulfur And Phosphorus.