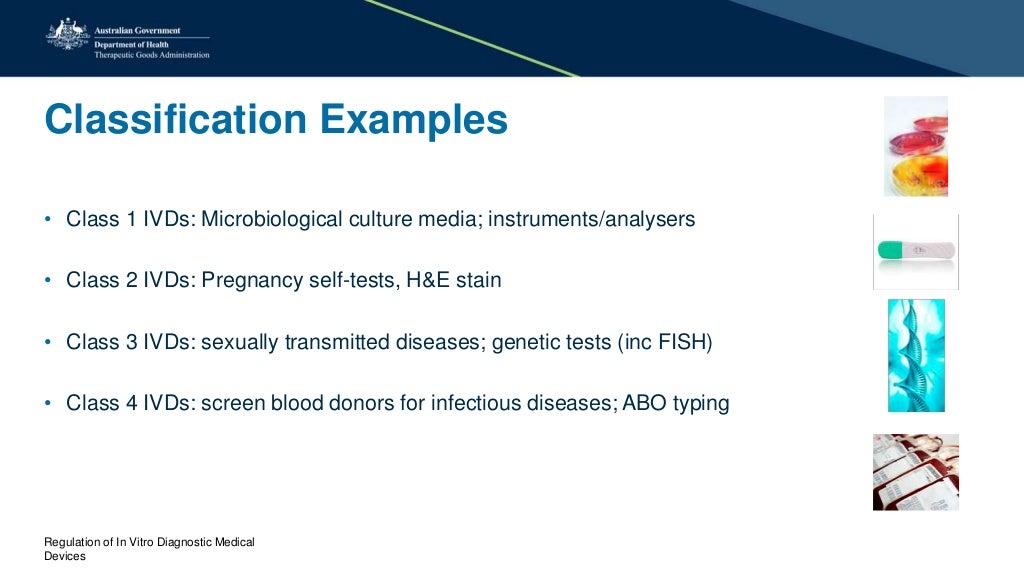
Non In Vitro Diagnostic Medical Devices . This document provides an internationally harmonized, modular, format for use when filing medical device submissions to regulatory. Estar is free and is required for all medical device 510 (k) submissions, unless exempted, to the center for devices and. This document outlines the current controls on the sale and supply of in vitro diagnostic (ivd) medical devices in great britain and explains. The in vitro diagnostic devices regulation applies since 26 may 2022. In vitro diagnostic products are those reagents, instruments, and systems intended for use in diagnosis of disease or other conditions,. It repeals directive 98/79/ec of the european parliament and of the council. In this guidance, “medical device” includes in vitro diagnostic medical devices and active implantable medical devices.
from www.slideshare.net
In vitro diagnostic products are those reagents, instruments, and systems intended for use in diagnosis of disease or other conditions,. Estar is free and is required for all medical device 510 (k) submissions, unless exempted, to the center for devices and. It repeals directive 98/79/ec of the european parliament and of the council. This document outlines the current controls on the sale and supply of in vitro diagnostic (ivd) medical devices in great britain and explains. This document provides an internationally harmonized, modular, format for use when filing medical device submissions to regulatory. The in vitro diagnostic devices regulation applies since 26 may 2022. In this guidance, “medical device” includes in vitro diagnostic medical devices and active implantable medical devices.
Regulation of In Vitro Diagnostic Medical Devices Transition to the…
Non In Vitro Diagnostic Medical Devices It repeals directive 98/79/ec of the european parliament and of the council. It repeals directive 98/79/ec of the european parliament and of the council. This document provides an internationally harmonized, modular, format for use when filing medical device submissions to regulatory. In vitro diagnostic products are those reagents, instruments, and systems intended for use in diagnosis of disease or other conditions,. In this guidance, “medical device” includes in vitro diagnostic medical devices and active implantable medical devices. Estar is free and is required for all medical device 510 (k) submissions, unless exempted, to the center for devices and. This document outlines the current controls on the sale and supply of in vitro diagnostic (ivd) medical devices in great britain and explains. The in vitro diagnostic devices regulation applies since 26 may 2022.
From management-forum.co.uk
Introduction to the InVitro Diagnostic Regulation (IVDR) Non In Vitro Diagnostic Medical Devices This document outlines the current controls on the sale and supply of in vitro diagnostic (ivd) medical devices in great britain and explains. This document provides an internationally harmonized, modular, format for use when filing medical device submissions to regulatory. In this guidance, “medical device” includes in vitro diagnostic medical devices and active implantable medical devices. Estar is free and. Non In Vitro Diagnostic Medical Devices.
From www.researchgate.net
List of FDACleared or Approved Companion Diagnostic Devices (In Vitro Non In Vitro Diagnostic Medical Devices In this guidance, “medical device” includes in vitro diagnostic medical devices and active implantable medical devices. It repeals directive 98/79/ec of the european parliament and of the council. This document provides an internationally harmonized, modular, format for use when filing medical device submissions to regulatory. The in vitro diagnostic devices regulation applies since 26 may 2022. This document outlines the. Non In Vitro Diagnostic Medical Devices.
From www.mdpi.com
A Systematic Database Approach to Identify Companion Diagnostic Testing Non In Vitro Diagnostic Medical Devices In this guidance, “medical device” includes in vitro diagnostic medical devices and active implantable medical devices. In vitro diagnostic products are those reagents, instruments, and systems intended for use in diagnosis of disease or other conditions,. Estar is free and is required for all medical device 510 (k) submissions, unless exempted, to the center for devices and. It repeals directive. Non In Vitro Diagnostic Medical Devices.
From www.euractiv.com
Commission presents guideline to avoid medical devices shortage Euractiv Non In Vitro Diagnostic Medical Devices This document provides an internationally harmonized, modular, format for use when filing medical device submissions to regulatory. Estar is free and is required for all medical device 510 (k) submissions, unless exempted, to the center for devices and. In vitro diagnostic products are those reagents, instruments, and systems intended for use in diagnosis of disease or other conditions,. This document. Non In Vitro Diagnostic Medical Devices.
From www.slideserve.com
PPT IVD and Point of care testing PowerPoint Presentation ID6646789 Non In Vitro Diagnostic Medical Devices This document outlines the current controls on the sale and supply of in vitro diagnostic (ivd) medical devices in great britain and explains. Estar is free and is required for all medical device 510 (k) submissions, unless exempted, to the center for devices and. This document provides an internationally harmonized, modular, format for use when filing medical device submissions to. Non In Vitro Diagnostic Medical Devices.
From cliniexperts.com
Why Do You Need InVitro Diagnostic Kits (IVD) Import License Non In Vitro Diagnostic Medical Devices In vitro diagnostic products are those reagents, instruments, and systems intended for use in diagnosis of disease or other conditions,. This document provides an internationally harmonized, modular, format for use when filing medical device submissions to regulatory. The in vitro diagnostic devices regulation applies since 26 may 2022. In this guidance, “medical device” includes in vitro diagnostic medical devices and. Non In Vitro Diagnostic Medical Devices.
From www.slideshare.net
Regulation of In Vitro Diagnostic Medical Devices Transition to the… Non In Vitro Diagnostic Medical Devices In vitro diagnostic products are those reagents, instruments, and systems intended for use in diagnosis of disease or other conditions,. This document outlines the current controls on the sale and supply of in vitro diagnostic (ivd) medical devices in great britain and explains. This document provides an internationally harmonized, modular, format for use when filing medical device submissions to regulatory.. Non In Vitro Diagnostic Medical Devices.
From www.frontiersin.org
Frontiers Advanced Biomimetic Nanomaterials for Noninvasive Disease Non In Vitro Diagnostic Medical Devices In vitro diagnostic products are those reagents, instruments, and systems intended for use in diagnosis of disease or other conditions,. This document outlines the current controls on the sale and supply of in vitro diagnostic (ivd) medical devices in great britain and explains. Estar is free and is required for all medical device 510 (k) submissions, unless exempted, to the. Non In Vitro Diagnostic Medical Devices.
From www.nsf.org
In Vitro Diagnostic Medical Device Regulation… NSF International Non In Vitro Diagnostic Medical Devices In vitro diagnostic products are those reagents, instruments, and systems intended for use in diagnosis of disease or other conditions,. The in vitro diagnostic devices regulation applies since 26 may 2022. This document outlines the current controls on the sale and supply of in vitro diagnostic (ivd) medical devices in great britain and explains. In this guidance, “medical device” includes. Non In Vitro Diagnostic Medical Devices.
From cliniexperts.com
CDSCO Recently Classified 80 InVitro Diagnostic Medical Devices For Non In Vitro Diagnostic Medical Devices The in vitro diagnostic devices regulation applies since 26 may 2022. This document outlines the current controls on the sale and supply of in vitro diagnostic (ivd) medical devices in great britain and explains. This document provides an internationally harmonized, modular, format for use when filing medical device submissions to regulatory. It repeals directive 98/79/ec of the european parliament and. Non In Vitro Diagnostic Medical Devices.
From riskandcompliance.freshfields.com
New EU Framework for In Vitro Diagnostic Medical Devices are you Non In Vitro Diagnostic Medical Devices The in vitro diagnostic devices regulation applies since 26 may 2022. Estar is free and is required for all medical device 510 (k) submissions, unless exempted, to the center for devices and. In vitro diagnostic products are those reagents, instruments, and systems intended for use in diagnosis of disease or other conditions,. It repeals directive 98/79/ec of the european parliament. Non In Vitro Diagnostic Medical Devices.
From studylib.net
NonIn Vitro Diagnostic Device Market Authorization Table of Contents Non In Vitro Diagnostic Medical Devices This document outlines the current controls on the sale and supply of in vitro diagnostic (ivd) medical devices in great britain and explains. In vitro diagnostic products are those reagents, instruments, and systems intended for use in diagnosis of disease or other conditions,. This document provides an internationally harmonized, modular, format for use when filing medical device submissions to regulatory.. Non In Vitro Diagnostic Medical Devices.
From apacmed.org
What Is In Vitro Diagnostics (IVD) Types, Benefits & Regulations Non In Vitro Diagnostic Medical Devices In this guidance, “medical device” includes in vitro diagnostic medical devices and active implantable medical devices. This document outlines the current controls on the sale and supply of in vitro diagnostic (ivd) medical devices in great britain and explains. Estar is free and is required for all medical device 510 (k) submissions, unless exempted, to the center for devices and.. Non In Vitro Diagnostic Medical Devices.
From mdrc-consulting.com
Europe's IVD regulatory approval process MDRC Non In Vitro Diagnostic Medical Devices In this guidance, “medical device” includes in vitro diagnostic medical devices and active implantable medical devices. The in vitro diagnostic devices regulation applies since 26 may 2022. It repeals directive 98/79/ec of the european parliament and of the council. This document provides an internationally harmonized, modular, format for use when filing medical device submissions to regulatory. In vitro diagnostic products. Non In Vitro Diagnostic Medical Devices.
From dicentra.com
EU In Vitro Diagnostic Medical Device Regulation dicentra Non In Vitro Diagnostic Medical Devices This document provides an internationally harmonized, modular, format for use when filing medical device submissions to regulatory. This document outlines the current controls on the sale and supply of in vitro diagnostic (ivd) medical devices in great britain and explains. In vitro diagnostic products are those reagents, instruments, and systems intended for use in diagnosis of disease or other conditions,.. Non In Vitro Diagnostic Medical Devices.
From www.thema-med.com
In Vitro Diagnostic Medical Devices proposed extension of IVDR 2017/746 Non In Vitro Diagnostic Medical Devices This document outlines the current controls on the sale and supply of in vitro diagnostic (ivd) medical devices in great britain and explains. It repeals directive 98/79/ec of the european parliament and of the council. In this guidance, “medical device” includes in vitro diagnostic medical devices and active implantable medical devices. This document provides an internationally harmonized, modular, format for. Non In Vitro Diagnostic Medical Devices.
From www.qvccert.com
InVitro Diagnostic Medical Devices CE Marking CE Marking, Ce Mark Non In Vitro Diagnostic Medical Devices This document outlines the current controls on the sale and supply of in vitro diagnostic (ivd) medical devices in great britain and explains. This document provides an internationally harmonized, modular, format for use when filing medical device submissions to regulatory. Estar is free and is required for all medical device 510 (k) submissions, unless exempted, to the center for devices. Non In Vitro Diagnostic Medical Devices.
From www.tuvsud.com
IVDR Infographic TÜV SÜD Non In Vitro Diagnostic Medical Devices The in vitro diagnostic devices regulation applies since 26 may 2022. It repeals directive 98/79/ec of the european parliament and of the council. Estar is free and is required for all medical device 510 (k) submissions, unless exempted, to the center for devices and. In this guidance, “medical device” includes in vitro diagnostic medical devices and active implantable medical devices.. Non In Vitro Diagnostic Medical Devices.
From qbdgroup.com
IVDR classification of invitro diagnostic medical devices a brief guide Non In Vitro Diagnostic Medical Devices Estar is free and is required for all medical device 510 (k) submissions, unless exempted, to the center for devices and. This document outlines the current controls on the sale and supply of in vitro diagnostic (ivd) medical devices in great britain and explains. In vitro diagnostic products are those reagents, instruments, and systems intended for use in diagnosis of. Non In Vitro Diagnostic Medical Devices.
From www.ethiobiomedical.com
Guidelines for Classification of Medical devices other than In Vitro Non In Vitro Diagnostic Medical Devices This document outlines the current controls on the sale and supply of in vitro diagnostic (ivd) medical devices in great britain and explains. In this guidance, “medical device” includes in vitro diagnostic medical devices and active implantable medical devices. This document provides an internationally harmonized, modular, format for use when filing medical device submissions to regulatory. Estar is free and. Non In Vitro Diagnostic Medical Devices.
From www.arc-regulatory.com
InVitro Diagnostics (IVD) & Medical Device Expertise Consulting ARC Non In Vitro Diagnostic Medical Devices In this guidance, “medical device” includes in vitro diagnostic medical devices and active implantable medical devices. In vitro diagnostic products are those reagents, instruments, and systems intended for use in diagnosis of disease or other conditions,. This document outlines the current controls on the sale and supply of in vitro diagnostic (ivd) medical devices in great britain and explains. This. Non In Vitro Diagnostic Medical Devices.
From www.canada.ca
Guidance Document Guidance on the Riskbased Classification System Non In Vitro Diagnostic Medical Devices Estar is free and is required for all medical device 510 (k) submissions, unless exempted, to the center for devices and. This document outlines the current controls on the sale and supply of in vitro diagnostic (ivd) medical devices in great britain and explains. This document provides an internationally harmonized, modular, format for use when filing medical device submissions to. Non In Vitro Diagnostic Medical Devices.
From www.pewtrusts.org
What Are In Vitro Diagnostic Tests, and How Are They Regulated? The Non In Vitro Diagnostic Medical Devices It repeals directive 98/79/ec of the european parliament and of the council. In vitro diagnostic products are those reagents, instruments, and systems intended for use in diagnosis of disease or other conditions,. The in vitro diagnostic devices regulation applies since 26 may 2022. This document provides an internationally harmonized, modular, format for use when filing medical device submissions to regulatory.. Non In Vitro Diagnostic Medical Devices.
From www.tuvsud.cn
Infographic The In Vitro Diagnostic Medical Device Regulation TÜV南德 Non In Vitro Diagnostic Medical Devices In vitro diagnostic products are those reagents, instruments, and systems intended for use in diagnosis of disease or other conditions,. It repeals directive 98/79/ec of the european parliament and of the council. The in vitro diagnostic devices regulation applies since 26 may 2022. This document provides an internationally harmonized, modular, format for use when filing medical device submissions to regulatory.. Non In Vitro Diagnostic Medical Devices.
From www.canada.ca
Guidance Document Guidance on the Riskbased Classification System Non In Vitro Diagnostic Medical Devices It repeals directive 98/79/ec of the european parliament and of the council. In vitro diagnostic products are those reagents, instruments, and systems intended for use in diagnosis of disease or other conditions,. In this guidance, “medical device” includes in vitro diagnostic medical devices and active implantable medical devices. This document outlines the current controls on the sale and supply of. Non In Vitro Diagnostic Medical Devices.
From www.linkedin.com
Draft Notifications for Classification of Non Notified InVitro Non In Vitro Diagnostic Medical Devices This document outlines the current controls on the sale and supply of in vitro diagnostic (ivd) medical devices in great britain and explains. Estar is free and is required for all medical device 510 (k) submissions, unless exempted, to the center for devices and. In vitro diagnostic products are those reagents, instruments, and systems intended for use in diagnosis of. Non In Vitro Diagnostic Medical Devices.
From www.clinicalresearchassociatecra.com
IVD Clinical Trials & Development Non In Vitro Diagnostic Medical Devices Estar is free and is required for all medical device 510 (k) submissions, unless exempted, to the center for devices and. The in vitro diagnostic devices regulation applies since 26 may 2022. This document provides an internationally harmonized, modular, format for use when filing medical device submissions to regulatory. It repeals directive 98/79/ec of the european parliament and of the. Non In Vitro Diagnostic Medical Devices.
From laegemiddelstyrelsen.dk
Classification of in vitro diagnostic medical devices (IVD) Non In Vitro Diagnostic Medical Devices The in vitro diagnostic devices regulation applies since 26 may 2022. Estar is free and is required for all medical device 510 (k) submissions, unless exempted, to the center for devices and. This document provides an internationally harmonized, modular, format for use when filing medical device submissions to regulatory. In vitro diagnostic products are those reagents, instruments, and systems intended. Non In Vitro Diagnostic Medical Devices.
From operonstrategist.com
Guide to In Vitro Diagnostic Medical Device Regulation (IVDR) IVD Non In Vitro Diagnostic Medical Devices This document outlines the current controls on the sale and supply of in vitro diagnostic (ivd) medical devices in great britain and explains. Estar is free and is required for all medical device 510 (k) submissions, unless exempted, to the center for devices and. In vitro diagnostic products are those reagents, instruments, and systems intended for use in diagnosis of. Non In Vitro Diagnostic Medical Devices.
From www.rcainc.com
Issues relevant for medical devices other than in vitro diagnostic Non In Vitro Diagnostic Medical Devices In this guidance, “medical device” includes in vitro diagnostic medical devices and active implantable medical devices. In vitro diagnostic products are those reagents, instruments, and systems intended for use in diagnosis of disease or other conditions,. Estar is free and is required for all medical device 510 (k) submissions, unless exempted, to the center for devices and. It repeals directive. Non In Vitro Diagnostic Medical Devices.
From www.canada.ca
Guidance Document Guidance on the Riskbased Classification System Non In Vitro Diagnostic Medical Devices This document provides an internationally harmonized, modular, format for use when filing medical device submissions to regulatory. The in vitro diagnostic devices regulation applies since 26 may 2022. This document outlines the current controls on the sale and supply of in vitro diagnostic (ivd) medical devices in great britain and explains. Estar is free and is required for all medical. Non In Vitro Diagnostic Medical Devices.
From www.regdesk.co
FDA Guidance on Labeling for In Vitro Diagnostic Devices RegDesk Non In Vitro Diagnostic Medical Devices The in vitro diagnostic devices regulation applies since 26 may 2022. In vitro diagnostic products are those reagents, instruments, and systems intended for use in diagnosis of disease or other conditions,. This document outlines the current controls on the sale and supply of in vitro diagnostic (ivd) medical devices in great britain and explains. This document provides an internationally harmonized,. Non In Vitro Diagnostic Medical Devices.
From lsacademy.com
Performance Evaluation of InVitroDiagnostic Devices (IVDs) LS Academy Non In Vitro Diagnostic Medical Devices This document outlines the current controls on the sale and supply of in vitro diagnostic (ivd) medical devices in great britain and explains. The in vitro diagnostic devices regulation applies since 26 may 2022. It repeals directive 98/79/ec of the european parliament and of the council. Estar is free and is required for all medical device 510 (k) submissions, unless. Non In Vitro Diagnostic Medical Devices.
From www.psi-software.com
In Vitro Diagnostics (IVD) Device Precision Systems, Inc. Non In Vitro Diagnostic Medical Devices This document outlines the current controls on the sale and supply of in vitro diagnostic (ivd) medical devices in great britain and explains. Estar is free and is required for all medical device 510 (k) submissions, unless exempted, to the center for devices and. This document provides an internationally harmonized, modular, format for use when filing medical device submissions to. Non In Vitro Diagnostic Medical Devices.
From stock.adobe.com
In vitro diagnostic medical device symbol. Stock Vector Adobe Stock Non In Vitro Diagnostic Medical Devices In this guidance, “medical device” includes in vitro diagnostic medical devices and active implantable medical devices. This document provides an internationally harmonized, modular, format for use when filing medical device submissions to regulatory. The in vitro diagnostic devices regulation applies since 26 may 2022. It repeals directive 98/79/ec of the european parliament and of the council. Estar is free and. Non In Vitro Diagnostic Medical Devices.