Characteristics Of Coordinate Bond . It is also called a dative bond or dipolar bond. The carbon monoxide molecule is correctly represented by a triple covalent bond between the carbon and oxygen atoms. One of the bonds is. In a coordinate covalent bond, the attraction of both nuclei to the electron pair holds the bond together. A coordinate bond (also called a dative covalent bond) is a covalent bond (a shared pair of electrons) in which both electrons come from. Both shared electrons are donated by the same atom. A coordinate bond is a type of covalent bond where both of the electrons that form the bond originate from the same atom (more generally, a dative covalent bond). Coordinate covalent bonds form in lewis acid and base reactions, metal ions bonding to ligands, and sometimes between nonmetals. Characteristics of coordinate covalent bond in coordinate bonding, the donor is the atom that shares a pair of electrons from itself to the.
from asiasupergrid.com
The carbon monoxide molecule is correctly represented by a triple covalent bond between the carbon and oxygen atoms. It is also called a dative bond or dipolar bond. In a coordinate covalent bond, the attraction of both nuclei to the electron pair holds the bond together. One of the bonds is. A coordinate bond is a type of covalent bond where both of the electrons that form the bond originate from the same atom (more generally, a dative covalent bond). Characteristics of coordinate covalent bond in coordinate bonding, the donor is the atom that shares a pair of electrons from itself to the. Coordinate covalent bonds form in lewis acid and base reactions, metal ions bonding to ligands, and sometimes between nonmetals. Both shared electrons are donated by the same atom. A coordinate bond (also called a dative covalent bond) is a covalent bond (a shared pair of electrons) in which both electrons come from.
Coordinate Covalent Bond Lewis Example
Characteristics Of Coordinate Bond One of the bonds is. Characteristics of coordinate covalent bond in coordinate bonding, the donor is the atom that shares a pair of electrons from itself to the. The carbon monoxide molecule is correctly represented by a triple covalent bond between the carbon and oxygen atoms. One of the bonds is. A coordinate bond is a type of covalent bond where both of the electrons that form the bond originate from the same atom (more generally, a dative covalent bond). It is also called a dative bond or dipolar bond. A coordinate bond (also called a dative covalent bond) is a covalent bond (a shared pair of electrons) in which both electrons come from. Both shared electrons are donated by the same atom. Coordinate covalent bonds form in lewis acid and base reactions, metal ions bonding to ligands, and sometimes between nonmetals. In a coordinate covalent bond, the attraction of both nuclei to the electron pair holds the bond together.
From pediaa.com
Difference Between Covalent and Coordinate Bond Definition, Formation Characteristics Of Coordinate Bond One of the bonds is. A coordinate bond (also called a dative covalent bond) is a covalent bond (a shared pair of electrons) in which both electrons come from. Both shared electrons are donated by the same atom. Characteristics of coordinate covalent bond in coordinate bonding, the donor is the atom that shares a pair of electrons from itself to. Characteristics Of Coordinate Bond.
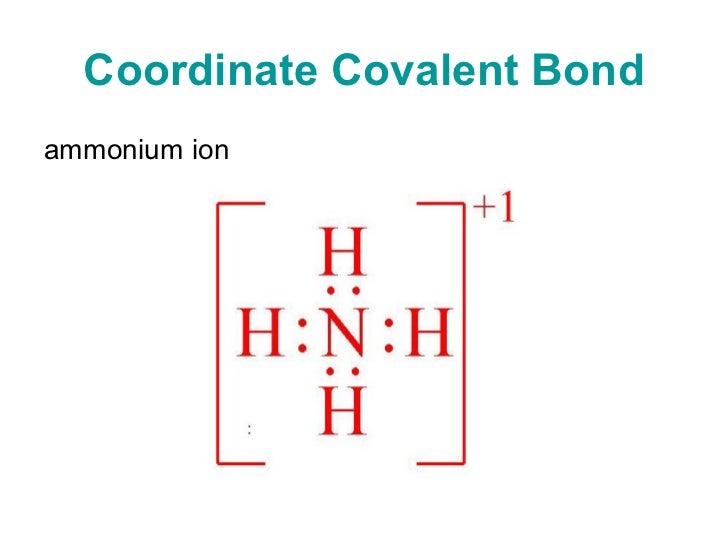
From www.slideserve.com
PPT Chemical Bonding 4 IONIC, METALLIC & COORDINATE BONDS PowerPoint Characteristics Of Coordinate Bond In a coordinate covalent bond, the attraction of both nuclei to the electron pair holds the bond together. It is also called a dative bond or dipolar bond. One of the bonds is. Characteristics of coordinate covalent bond in coordinate bonding, the donor is the atom that shares a pair of electrons from itself to the. Both shared electrons are. Characteristics Of Coordinate Bond.
From www.youtube.com
How Coordinate Bond (Dative Covalent Bond) is Formed? YouTube Characteristics Of Coordinate Bond Coordinate covalent bonds form in lewis acid and base reactions, metal ions bonding to ligands, and sometimes between nonmetals. In a coordinate covalent bond, the attraction of both nuclei to the electron pair holds the bond together. The carbon monoxide molecule is correctly represented by a triple covalent bond between the carbon and oxygen atoms. One of the bonds is.. Characteristics Of Coordinate Bond.
From thescienceandmathszone.com
Dative Covalent (Coordinate) Bonding The Science and Maths Zone Characteristics Of Coordinate Bond In a coordinate covalent bond, the attraction of both nuclei to the electron pair holds the bond together. A coordinate bond is a type of covalent bond where both of the electrons that form the bond originate from the same atom (more generally, a dative covalent bond). It is also called a dative bond or dipolar bond. Coordinate covalent bonds. Characteristics Of Coordinate Bond.
From www.chemistrylearner.com
Covalent Bond Definition, Types, and Examples Characteristics Of Coordinate Bond It is also called a dative bond or dipolar bond. One of the bonds is. Coordinate covalent bonds form in lewis acid and base reactions, metal ions bonding to ligands, and sometimes between nonmetals. The carbon monoxide molecule is correctly represented by a triple covalent bond between the carbon and oxygen atoms. A coordinate bond is a type of covalent. Characteristics Of Coordinate Bond.
From www.studypool.com
SOLUTION Chemistry coordinate bond diagram Studypool Characteristics Of Coordinate Bond The carbon monoxide molecule is correctly represented by a triple covalent bond between the carbon and oxygen atoms. In a coordinate covalent bond, the attraction of both nuclei to the electron pair holds the bond together. One of the bonds is. It is also called a dative bond or dipolar bond. A coordinate bond is a type of covalent bond. Characteristics Of Coordinate Bond.
From www.studypool.com
SOLUTION Chemistry coordinate bond diagram Studypool Characteristics Of Coordinate Bond The carbon monoxide molecule is correctly represented by a triple covalent bond between the carbon and oxygen atoms. One of the bonds is. Characteristics of coordinate covalent bond in coordinate bonding, the donor is the atom that shares a pair of electrons from itself to the. In a coordinate covalent bond, the attraction of both nuclei to the electron pair. Characteristics Of Coordinate Bond.
From www.youtube.com
What is a Coordinate Covalent Bond? YouTube Characteristics Of Coordinate Bond One of the bonds is. Characteristics of coordinate covalent bond in coordinate bonding, the donor is the atom that shares a pair of electrons from itself to the. Coordinate covalent bonds form in lewis acid and base reactions, metal ions bonding to ligands, and sometimes between nonmetals. A coordinate bond (also called a dative covalent bond) is a covalent bond. Characteristics Of Coordinate Bond.
From mydigitalkemistry.com
What is Coordinate Covalent Bond in Simple Words? Characteristics Of Coordinate Bond One of the bonds is. Both shared electrons are donated by the same atom. A coordinate bond (also called a dative covalent bond) is a covalent bond (a shared pair of electrons) in which both electrons come from. Coordinate covalent bonds form in lewis acid and base reactions, metal ions bonding to ligands, and sometimes between nonmetals. Characteristics of coordinate. Characteristics Of Coordinate Bond.
From www.youtube.com
Coordinate covalent bond or Dative Bond, Characteristics of coordinate Characteristics Of Coordinate Bond A coordinate bond is a type of covalent bond where both of the electrons that form the bond originate from the same atom (more generally, a dative covalent bond). A coordinate bond (also called a dative covalent bond) is a covalent bond (a shared pair of electrons) in which both electrons come from. Both shared electrons are donated by the. Characteristics Of Coordinate Bond.
From www.slideserve.com
PPT Chapter 10 Chemical Bonding I Basic Concepts PowerPoint Characteristics Of Coordinate Bond A coordinate bond is a type of covalent bond where both of the electrons that form the bond originate from the same atom (more generally, a dative covalent bond). Characteristics of coordinate covalent bond in coordinate bonding, the donor is the atom that shares a pair of electrons from itself to the. The carbon monoxide molecule is correctly represented by. Characteristics Of Coordinate Bond.
From www.studypool.com
SOLUTION Coordinate covalent bond Studypool Characteristics Of Coordinate Bond Both shared electrons are donated by the same atom. It is also called a dative bond or dipolar bond. A coordinate bond (also called a dative covalent bond) is a covalent bond (a shared pair of electrons) in which both electrons come from. In a coordinate covalent bond, the attraction of both nuclei to the electron pair holds the bond. Characteristics Of Coordinate Bond.
From www.slideserve.com
PPT Polar, Coordinate, & Network Covalent Bonds PowerPoint Characteristics Of Coordinate Bond A coordinate bond is a type of covalent bond where both of the electrons that form the bond originate from the same atom (more generally, a dative covalent bond). A coordinate bond (also called a dative covalent bond) is a covalent bond (a shared pair of electrons) in which both electrons come from. In a coordinate covalent bond, the attraction. Characteristics Of Coordinate Bond.
From www.slideserve.com
PPT Chemical Bonding PowerPoint Presentation, free download ID1989892 Characteristics Of Coordinate Bond A coordinate bond is a type of covalent bond where both of the electrons that form the bond originate from the same atom (more generally, a dative covalent bond). One of the bonds is. In a coordinate covalent bond, the attraction of both nuclei to the electron pair holds the bond together. Coordinate covalent bonds form in lewis acid and. Characteristics Of Coordinate Bond.
From thescienceandmathszone.com
Dative Covalent (Coordinate) Bonding The Science and Maths Zone Characteristics Of Coordinate Bond One of the bonds is. It is also called a dative bond or dipolar bond. Characteristics of coordinate covalent bond in coordinate bonding, the donor is the atom that shares a pair of electrons from itself to the. A coordinate bond is a type of covalent bond where both of the electrons that form the bond originate from the same. Characteristics Of Coordinate Bond.
From www.studypool.com
SOLUTION Explain coordinate covalent bond discuss types of bonds and Characteristics Of Coordinate Bond Both shared electrons are donated by the same atom. A coordinate bond is a type of covalent bond where both of the electrons that form the bond originate from the same atom (more generally, a dative covalent bond). Characteristics of coordinate covalent bond in coordinate bonding, the donor is the atom that shares a pair of electrons from itself to. Characteristics Of Coordinate Bond.
From asiasupergrid.com
Coordinate Covalent Bond Lewis Example Characteristics Of Coordinate Bond It is also called a dative bond or dipolar bond. Characteristics of coordinate covalent bond in coordinate bonding, the donor is the atom that shares a pair of electrons from itself to the. A coordinate bond is a type of covalent bond where both of the electrons that form the bond originate from the same atom (more generally, a dative. Characteristics Of Coordinate Bond.
From byjus.com
What is coordinated bond? Characteristics Of Coordinate Bond A coordinate bond (also called a dative covalent bond) is a covalent bond (a shared pair of electrons) in which both electrons come from. Both shared electrons are donated by the same atom. Coordinate covalent bonds form in lewis acid and base reactions, metal ions bonding to ligands, and sometimes between nonmetals. One of the bonds is. The carbon monoxide. Characteristics Of Coordinate Bond.
From www.studypool.com
SOLUTION Explain coordinate covalent bond discuss types of bonds and Characteristics Of Coordinate Bond In a coordinate covalent bond, the attraction of both nuclei to the electron pair holds the bond together. Characteristics of coordinate covalent bond in coordinate bonding, the donor is the atom that shares a pair of electrons from itself to the. It is also called a dative bond or dipolar bond. A coordinate bond is a type of covalent bond. Characteristics Of Coordinate Bond.
From www.slideserve.com
PPT Chemical Bonding PowerPoint Presentation ID1588665 Characteristics Of Coordinate Bond It is also called a dative bond or dipolar bond. In a coordinate covalent bond, the attraction of both nuclei to the electron pair holds the bond together. A coordinate bond is a type of covalent bond where both of the electrons that form the bond originate from the same atom (more generally, a dative covalent bond). One of the. Characteristics Of Coordinate Bond.
From www.slideserve.com
PPT Chapter 16 Covalent Bonding PowerPoint Presentation, free Characteristics Of Coordinate Bond Both shared electrons are donated by the same atom. A coordinate bond is a type of covalent bond where both of the electrons that form the bond originate from the same atom (more generally, a dative covalent bond). One of the bonds is. Coordinate covalent bonds form in lewis acid and base reactions, metal ions bonding to ligands, and sometimes. Characteristics Of Coordinate Bond.
From www.pinterest.com
Coordinate Covalent Bond Dative Bond Boron Atom, Ionic Compound Characteristics Of Coordinate Bond The carbon monoxide molecule is correctly represented by a triple covalent bond between the carbon and oxygen atoms. In a coordinate covalent bond, the attraction of both nuclei to the electron pair holds the bond together. Both shared electrons are donated by the same atom. One of the bonds is. Coordinate covalent bonds form in lewis acid and base reactions,. Characteristics Of Coordinate Bond.
From www.slideserve.com
PPT Topic 9 Chemical Bonding and Lewis Structures PowerPoint Characteristics Of Coordinate Bond In a coordinate covalent bond, the attraction of both nuclei to the electron pair holds the bond together. A coordinate bond (also called a dative covalent bond) is a covalent bond (a shared pair of electrons) in which both electrons come from. The carbon monoxide molecule is correctly represented by a triple covalent bond between the carbon and oxygen atoms.. Characteristics Of Coordinate Bond.
From www.studypool.com
SOLUTION Chemistry coordinate bond diagram Studypool Characteristics Of Coordinate Bond In a coordinate covalent bond, the attraction of both nuclei to the electron pair holds the bond together. Both shared electrons are donated by the same atom. A coordinate bond (also called a dative covalent bond) is a covalent bond (a shared pair of electrons) in which both electrons come from. Characteristics of coordinate covalent bond in coordinate bonding, the. Characteristics Of Coordinate Bond.
From www.youtube.com
Lewis dot structure /CO, HCl, NH3, NH4+, CO3^2_, AlCl3, Coordinate bond Characteristics Of Coordinate Bond Both shared electrons are donated by the same atom. A coordinate bond (also called a dative covalent bond) is a covalent bond (a shared pair of electrons) in which both electrons come from. One of the bonds is. The carbon monoxide molecule is correctly represented by a triple covalent bond between the carbon and oxygen atoms. A coordinate bond is. Characteristics Of Coordinate Bond.
From www.youtube.com
Coordination Compounds Geometry and Nomenclature YouTube Characteristics Of Coordinate Bond In a coordinate covalent bond, the attraction of both nuclei to the electron pair holds the bond together. One of the bonds is. Both shared electrons are donated by the same atom. A coordinate bond is a type of covalent bond where both of the electrons that form the bond originate from the same atom (more generally, a dative covalent. Characteristics Of Coordinate Bond.
From www.slideserve.com
PPT Chapter 8 PowerPoint Presentation, free download ID2372206 Characteristics Of Coordinate Bond In a coordinate covalent bond, the attraction of both nuclei to the electron pair holds the bond together. A coordinate bond is a type of covalent bond where both of the electrons that form the bond originate from the same atom (more generally, a dative covalent bond). Characteristics of coordinate covalent bond in coordinate bonding, the donor is the atom. Characteristics Of Coordinate Bond.
From thechemistrynotes.com
Coordinate Covalent Bond [Dative Covalent Bonding] Characteristics Of Coordinate Bond Coordinate covalent bonds form in lewis acid and base reactions, metal ions bonding to ligands, and sometimes between nonmetals. Characteristics of coordinate covalent bond in coordinate bonding, the donor is the atom that shares a pair of electrons from itself to the. In a coordinate covalent bond, the attraction of both nuclei to the electron pair holds the bond together.. Characteristics Of Coordinate Bond.
From www.pinterest.com
Coordinate Covalent Bond Definition , Examples ,Formation and Characteristics Of Coordinate Bond Both shared electrons are donated by the same atom. Characteristics of coordinate covalent bond in coordinate bonding, the donor is the atom that shares a pair of electrons from itself to the. It is also called a dative bond or dipolar bond. A coordinate bond is a type of covalent bond where both of the electrons that form the bond. Characteristics Of Coordinate Bond.
From www.youtube.com
What is a coordinate Bond? Examples , Properties , Types , and Characteristics Of Coordinate Bond A coordinate bond (also called a dative covalent bond) is a covalent bond (a shared pair of electrons) in which both electrons come from. The carbon monoxide molecule is correctly represented by a triple covalent bond between the carbon and oxygen atoms. It is also called a dative bond or dipolar bond. In a coordinate covalent bond, the attraction of. Characteristics Of Coordinate Bond.
From www.youtube.com
Chemical BondingCoordinate Covalent Bond YouTube Characteristics Of Coordinate Bond The carbon monoxide molecule is correctly represented by a triple covalent bond between the carbon and oxygen atoms. Characteristics of coordinate covalent bond in coordinate bonding, the donor is the atom that shares a pair of electrons from itself to the. It is also called a dative bond or dipolar bond. A coordinate bond (also called a dative covalent bond). Characteristics Of Coordinate Bond.
From www.bartleby.com
Coordinate Covalent Bonds bartleby Characteristics Of Coordinate Bond Characteristics of coordinate covalent bond in coordinate bonding, the donor is the atom that shares a pair of electrons from itself to the. A coordinate bond is a type of covalent bond where both of the electrons that form the bond originate from the same atom (more generally, a dative covalent bond). Both shared electrons are donated by the same. Characteristics Of Coordinate Bond.
From mydigitalkemistry.com
coordinate bond examples Free Chemistry Learning Platform Characteristics Of Coordinate Bond It is also called a dative bond or dipolar bond. Coordinate covalent bonds form in lewis acid and base reactions, metal ions bonding to ligands, and sometimes between nonmetals. A coordinate bond (also called a dative covalent bond) is a covalent bond (a shared pair of electrons) in which both electrons come from. The carbon monoxide molecule is correctly represented. Characteristics Of Coordinate Bond.
From www.chemistrylearner.com
Coordinate Covalent Bond Definition and Examples Characteristics Of Coordinate Bond Both shared electrons are donated by the same atom. In a coordinate covalent bond, the attraction of both nuclei to the electron pair holds the bond together. A coordinate bond (also called a dative covalent bond) is a covalent bond (a shared pair of electrons) in which both electrons come from. One of the bonds is. It is also called. Characteristics Of Coordinate Bond.
From stock.adobe.com
A coordinate bond is represented by pointing from the donor atom to the Characteristics Of Coordinate Bond One of the bonds is. A coordinate bond is a type of covalent bond where both of the electrons that form the bond originate from the same atom (more generally, a dative covalent bond). A coordinate bond (also called a dative covalent bond) is a covalent bond (a shared pair of electrons) in which both electrons come from. Characteristics of. Characteristics Of Coordinate Bond.