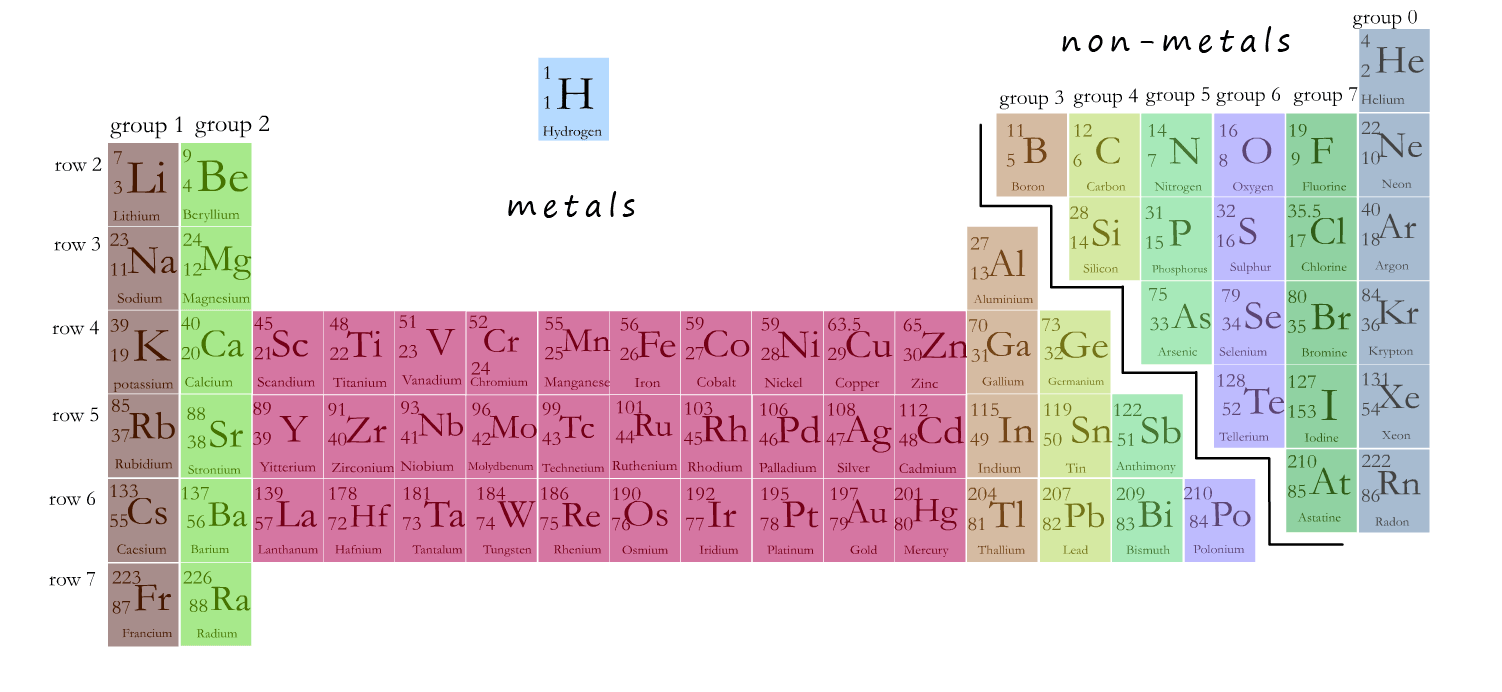Why Is The Bottom Of The Periodic Table Separated . The lanthanides and actinides are separated from the rest of the periodic table, usually appearing as separate rows at the bottom. The reason why lanthanides and actinides are located at the bottom of the periodical table is because of their properties and in the block in which electrons fill up. Both the lanthanide and actinide. The lanthanides and actinides are often separated from the main body of the periodic table to keep the table compact. Lanthanides and actinides are placed in separate rows at the bottom of the periodic table. They are also called inner transition elements. You mean to ask why the lanthanides and actinides are placed in a separate section of the periodic table. The reason for this placement has to do with the electron configurations of these elements. Give the reason for this arrangement. These rows contain elements in the lanthanoid. The periodic table has two rows at the bottom that are usually split out from the main body of the table. The two rows of elements present at the bottom of the periodic table are called the 4f series or lanthanoids and 5f or actanoids.
from www.science-revision.co.uk
The periodic table has two rows at the bottom that are usually split out from the main body of the table. The lanthanides and actinides are separated from the rest of the periodic table, usually appearing as separate rows at the bottom. Give the reason for this arrangement. The two rows of elements present at the bottom of the periodic table are called the 4f series or lanthanoids and 5f or actanoids. The lanthanides and actinides are often separated from the main body of the periodic table to keep the table compact. Lanthanides and actinides are placed in separate rows at the bottom of the periodic table. Both the lanthanide and actinide. These rows contain elements in the lanthanoid. The reason why lanthanides and actinides are located at the bottom of the periodical table is because of their properties and in the block in which electrons fill up. The reason for this placement has to do with the electron configurations of these elements.
Periodic table
Why Is The Bottom Of The Periodic Table Separated The periodic table has two rows at the bottom that are usually split out from the main body of the table. The reason why lanthanides and actinides are located at the bottom of the periodical table is because of their properties and in the block in which electrons fill up. Lanthanides and actinides are placed in separate rows at the bottom of the periodic table. Both the lanthanide and actinide. The periodic table has two rows at the bottom that are usually split out from the main body of the table. They are also called inner transition elements. The two rows of elements present at the bottom of the periodic table are called the 4f series or lanthanoids and 5f or actanoids. These rows contain elements in the lanthanoid. Give the reason for this arrangement. The lanthanides and actinides are often separated from the main body of the periodic table to keep the table compact. The lanthanides and actinides are separated from the rest of the periodic table, usually appearing as separate rows at the bottom. You mean to ask why the lanthanides and actinides are placed in a separate section of the periodic table. The reason for this placement has to do with the electron configurations of these elements.
From pressbooks.bccampus.ca
2.1 Elements and Atoms the Building Blocks of Matter Douglas College Why Is The Bottom Of The Periodic Table Separated They are also called inner transition elements. The lanthanides and actinides are often separated from the main body of the periodic table to keep the table compact. The two rows of elements present at the bottom of the periodic table are called the 4f series or lanthanoids and 5f or actanoids. These rows contain elements in the lanthanoid. The lanthanides. Why Is The Bottom Of The Periodic Table Separated.
From elchoroukhost.net
What Are The Bottom Two Rows Of Periodic Table Called Elcho Table Why Is The Bottom Of The Periodic Table Separated The lanthanides and actinides are separated from the rest of the periodic table, usually appearing as separate rows at the bottom. Both the lanthanide and actinide. The two rows of elements present at the bottom of the periodic table are called the 4f series or lanthanoids and 5f or actanoids. They are also called inner transition elements. The reason why. Why Is The Bottom Of The Periodic Table Separated.
From mmerevise.co.uk
The Periodic Table Questions and Revision MME Why Is The Bottom Of The Periodic Table Separated They are also called inner transition elements. Give the reason for this arrangement. The two rows of elements present at the bottom of the periodic table are called the 4f series or lanthanoids and 5f or actanoids. Lanthanides and actinides are placed in separate rows at the bottom of the periodic table. The lanthanides and actinides are separated from the. Why Is The Bottom Of The Periodic Table Separated.
From sciencenotes.org
Periodic Table Groups and Periods Why Is The Bottom Of The Periodic Table Separated You mean to ask why the lanthanides and actinides are placed in a separate section of the periodic table. They are also called inner transition elements. The two rows of elements present at the bottom of the periodic table are called the 4f series or lanthanoids and 5f or actanoids. Both the lanthanide and actinide. The lanthanides and actinides are. Why Is The Bottom Of The Periodic Table Separated.
From www.collegesearch.in
Periodic Table with Names Definitions, Characteristics, Properties Why Is The Bottom Of The Periodic Table Separated Both the lanthanide and actinide. Give the reason for this arrangement. The two rows of elements present at the bottom of the periodic table are called the 4f series or lanthanoids and 5f or actanoids. The lanthanides and actinides are separated from the rest of the periodic table, usually appearing as separate rows at the bottom. You mean to ask. Why Is The Bottom Of The Periodic Table Separated.
From elchoroukhost.net
Transition Elements Periodic Table Elcho Table Why Is The Bottom Of The Periodic Table Separated These rows contain elements in the lanthanoid. The two rows of elements present at the bottom of the periodic table are called the 4f series or lanthanoids and 5f or actanoids. They are also called inner transition elements. Both the lanthanide and actinide. The lanthanides and actinides are separated from the rest of the periodic table, usually appearing as separate. Why Is The Bottom Of The Periodic Table Separated.
From www.reddit.com
Why are the lanthanides and actinides removed from the rest of the Why Is The Bottom Of The Periodic Table Separated The lanthanides and actinides are separated from the rest of the periodic table, usually appearing as separate rows at the bottom. They are also called inner transition elements. The lanthanides and actinides are often separated from the main body of the periodic table to keep the table compact. Give the reason for this arrangement. Lanthanides and actinides are placed in. Why Is The Bottom Of The Periodic Table Separated.
From sciencenotes.org
Periodic Table Blocks of Elements Why Is The Bottom Of The Periodic Table Separated You mean to ask why the lanthanides and actinides are placed in a separate section of the periodic table. The periodic table has two rows at the bottom that are usually split out from the main body of the table. The two rows of elements present at the bottom of the periodic table are called the 4f series or lanthanoids. Why Is The Bottom Of The Periodic Table Separated.
From utedzz.blogspot.com
Periodic Table Orbitals Chemistry Periodic Table Timeline Why Is The Bottom Of The Periodic Table Separated Give the reason for this arrangement. Lanthanides and actinides are placed in separate rows at the bottom of the periodic table. Both the lanthanide and actinide. You mean to ask why the lanthanides and actinides are placed in a separate section of the periodic table. The periodic table has two rows at the bottom that are usually split out from. Why Is The Bottom Of The Periodic Table Separated.
From www.futurity.org
The periodic table is finally complete Futurity Why Is The Bottom Of The Periodic Table Separated They are also called inner transition elements. The reason for this placement has to do with the electron configurations of these elements. These rows contain elements in the lanthanoid. The two rows of elements present at the bottom of the periodic table are called the 4f series or lanthanoids and 5f or actanoids. The periodic table has two rows at. Why Is The Bottom Of The Periodic Table Separated.
From chem.libretexts.org
6.9 Electron Configurations and the Periodic Table Chemistry LibreTexts Why Is The Bottom Of The Periodic Table Separated The two rows of elements present at the bottom of the periodic table are called the 4f series or lanthanoids and 5f or actanoids. The lanthanides and actinides are often separated from the main body of the periodic table to keep the table compact. Give the reason for this arrangement. These rows contain elements in the lanthanoid. The periodic table. Why Is The Bottom Of The Periodic Table Separated.
From masterconceptsinchemistry.com
What’s the periodic table? Why Is The Bottom Of The Periodic Table Separated They are also called inner transition elements. The lanthanides and actinides are separated from the rest of the periodic table, usually appearing as separate rows at the bottom. Give the reason for this arrangement. You mean to ask why the lanthanides and actinides are placed in a separate section of the periodic table. These rows contain elements in the lanthanoid.. Why Is The Bottom Of The Periodic Table Separated.
From mungfali.com
Periodic Table Groupings Why Is The Bottom Of The Periodic Table Separated Both the lanthanide and actinide. They are also called inner transition elements. The reason why lanthanides and actinides are located at the bottom of the periodical table is because of their properties and in the block in which electrons fill up. The two rows of elements present at the bottom of the periodic table are called the 4f series or. Why Is The Bottom Of The Periodic Table Separated.
From cabinet.matttroy.net
Periodic Table Of Elements Solid Liquid Gas At Room Temperature Why Is The Bottom Of The Periodic Table Separated The reason for this placement has to do with the electron configurations of these elements. The two rows of elements present at the bottom of the periodic table are called the 4f series or lanthanoids and 5f or actanoids. The lanthanides and actinides are often separated from the main body of the periodic table to keep the table compact. You. Why Is The Bottom Of The Periodic Table Separated.
From nool.ontariotechu.ca
Periodic table nool Why Is The Bottom Of The Periodic Table Separated Both the lanthanide and actinide. Give the reason for this arrangement. Lanthanides and actinides are placed in separate rows at the bottom of the periodic table. The lanthanides and actinides are often separated from the main body of the periodic table to keep the table compact. You mean to ask why the lanthanides and actinides are placed in a separate. Why Is The Bottom Of The Periodic Table Separated.
From www.science-revision.co.uk
Periodic table Why Is The Bottom Of The Periodic Table Separated You mean to ask why the lanthanides and actinides are placed in a separate section of the periodic table. These rows contain elements in the lanthanoid. The lanthanides and actinides are separated from the rest of the periodic table, usually appearing as separate rows at the bottom. The two rows of elements present at the bottom of the periodic table. Why Is The Bottom Of The Periodic Table Separated.
From www.pearson.com
The most important parts of the periodic table for organic chemis Why Is The Bottom Of The Periodic Table Separated The periodic table has two rows at the bottom that are usually split out from the main body of the table. The lanthanides and actinides are often separated from the main body of the periodic table to keep the table compact. The reason why lanthanides and actinides are located at the bottom of the periodical table is because of their. Why Is The Bottom Of The Periodic Table Separated.
From nool.ontariotechu.ca
Periodic table nool Why Is The Bottom Of The Periodic Table Separated The lanthanides and actinides are often separated from the main body of the periodic table to keep the table compact. These rows contain elements in the lanthanoid. The periodic table has two rows at the bottom that are usually split out from the main body of the table. They are also called inner transition elements. Lanthanides and actinides are placed. Why Is The Bottom Of The Periodic Table Separated.
From chem.libretexts.org
2.6 The Periodic Table Chemistry LibreTexts Why Is The Bottom Of The Periodic Table Separated Give the reason for this arrangement. Lanthanides and actinides are placed in separate rows at the bottom of the periodic table. These rows contain elements in the lanthanoid. The lanthanides and actinides are often separated from the main body of the periodic table to keep the table compact. They are also called inner transition elements. The lanthanides and actinides are. Why Is The Bottom Of The Periodic Table Separated.
From www.britannica.com
periodic table Definition & Groups Britannica Why Is The Bottom Of The Periodic Table Separated The reason why lanthanides and actinides are located at the bottom of the periodical table is because of their properties and in the block in which electrons fill up. You mean to ask why the lanthanides and actinides are placed in a separate section of the periodic table. The reason for this placement has to do with the electron configurations. Why Is The Bottom Of The Periodic Table Separated.
From ecampusontario.pressbooks.pub
3.2 The Periodic Table Enhanced Introductory College Chemistry Why Is The Bottom Of The Periodic Table Separated These rows contain elements in the lanthanoid. The reason for this placement has to do with the electron configurations of these elements. Both the lanthanide and actinide. You mean to ask why the lanthanides and actinides are placed in a separate section of the periodic table. The periodic table has two rows at the bottom that are usually split out. Why Is The Bottom Of The Periodic Table Separated.
From edu.rsc.org
How to read the periodic table Poster RSC Education Why Is The Bottom Of The Periodic Table Separated These rows contain elements in the lanthanoid. The reason why lanthanides and actinides are located at the bottom of the periodical table is because of their properties and in the block in which electrons fill up. You mean to ask why the lanthanides and actinides are placed in a separate section of the periodic table. The reason for this placement. Why Is The Bottom Of The Periodic Table Separated.
From www.thesciencehive.co.uk
The Periodic Table (AQA) — the science hive Why Is The Bottom Of The Periodic Table Separated The reason for this placement has to do with the electron configurations of these elements. Both the lanthanide and actinide. You mean to ask why the lanthanides and actinides are placed in a separate section of the periodic table. The periodic table has two rows at the bottom that are usually split out from the main body of the table.. Why Is The Bottom Of The Periodic Table Separated.
From chem.libretexts.org
9.7 Electron Configurations and the Periodic Table Chemistry LibreTexts Why Is The Bottom Of The Periodic Table Separated Both the lanthanide and actinide. They are also called inner transition elements. Lanthanides and actinides are placed in separate rows at the bottom of the periodic table. The two rows of elements present at the bottom of the periodic table are called the 4f series or lanthanoids and 5f or actanoids. The lanthanides and actinides are often separated from the. Why Is The Bottom Of The Periodic Table Separated.
From elchoroukhost.net
What Are The Bottom Two Rows Of Periodic Table Called Elcho Table Why Is The Bottom Of The Periodic Table Separated These rows contain elements in the lanthanoid. The lanthanides and actinides are separated from the rest of the periodic table, usually appearing as separate rows at the bottom. You mean to ask why the lanthanides and actinides are placed in a separate section of the periodic table. Lanthanides and actinides are placed in separate rows at the bottom of the. Why Is The Bottom Of The Periodic Table Separated.
From utedzz.blogspot.com
Periodic Table With All Groups Labeled Periodic Table Timeline Why Is The Bottom Of The Periodic Table Separated You mean to ask why the lanthanides and actinides are placed in a separate section of the periodic table. The lanthanides and actinides are often separated from the main body of the periodic table to keep the table compact. The reason for this placement has to do with the electron configurations of these elements. Both the lanthanide and actinide. They. Why Is The Bottom Of The Periodic Table Separated.
From www.youtube.com
Periods and groups in the periodic table YouTube Why Is The Bottom Of The Periodic Table Separated You mean to ask why the lanthanides and actinides are placed in a separate section of the periodic table. The two rows of elements present at the bottom of the periodic table are called the 4f series or lanthanoids and 5f or actanoids. They are also called inner transition elements. The lanthanides and actinides are often separated from the main. Why Is The Bottom Of The Periodic Table Separated.
From resource.studiaacademy.com
edexcel_igcse_chemistry_topic04_theperiodictable_001_periodictable Why Is The Bottom Of The Periodic Table Separated The periodic table has two rows at the bottom that are usually split out from the main body of the table. The reason for this placement has to do with the electron configurations of these elements. The lanthanides and actinides are separated from the rest of the periodic table, usually appearing as separate rows at the bottom. Lanthanides and actinides. Why Is The Bottom Of The Periodic Table Separated.
From mavink.com
Periodic Table Split Into Blocks Why Is The Bottom Of The Periodic Table Separated Both the lanthanide and actinide. The lanthanides and actinides are separated from the rest of the periodic table, usually appearing as separate rows at the bottom. The lanthanides and actinides are often separated from the main body of the periodic table to keep the table compact. The periodic table has two rows at the bottom that are usually split out. Why Is The Bottom Of The Periodic Table Separated.
From school.careers360.com
How many Groups are in the Periodic Table Why Is The Bottom Of The Periodic Table Separated Give the reason for this arrangement. The periodic table has two rows at the bottom that are usually split out from the main body of the table. Lanthanides and actinides are placed in separate rows at the bottom of the periodic table. These rows contain elements in the lanthanoid. Both the lanthanide and actinide. The reason for this placement has. Why Is The Bottom Of The Periodic Table Separated.
From examples.yourdictionary.com
Basic Types of Metals on the Periodic Table Why Is The Bottom Of The Periodic Table Separated The reason for this placement has to do with the electron configurations of these elements. These rows contain elements in the lanthanoid. Give the reason for this arrangement. You mean to ask why the lanthanides and actinides are placed in a separate section of the periodic table. The periodic table has two rows at the bottom that are usually split. Why Is The Bottom Of The Periodic Table Separated.
From www.periodictableprintable.com
Periodic Table Of Elements With Group Numbers 2023 Periodic Table Why Is The Bottom Of The Periodic Table Separated Both the lanthanide and actinide. The periodic table has two rows at the bottom that are usually split out from the main body of the table. The reason for this placement has to do with the electron configurations of these elements. The lanthanides and actinides are often separated from the main body of the periodic table to keep the table. Why Is The Bottom Of The Periodic Table Separated.
From www.cambridge.org
Periodic table of the elements (Appendix B) Introduction to Why Is The Bottom Of The Periodic Table Separated Both the lanthanide and actinide. Give the reason for this arrangement. Lanthanides and actinides are placed in separate rows at the bottom of the periodic table. You mean to ask why the lanthanides and actinides are placed in a separate section of the periodic table. The reason why lanthanides and actinides are located at the bottom of the periodical table. Why Is The Bottom Of The Periodic Table Separated.
From www.thoughtco.com
Properties of Periodic Table of Element Groups Why Is The Bottom Of The Periodic Table Separated The lanthanides and actinides are separated from the rest of the periodic table, usually appearing as separate rows at the bottom. The reason for this placement has to do with the electron configurations of these elements. Lanthanides and actinides are placed in separate rows at the bottom of the periodic table. Both the lanthanide and actinide. The periodic table has. Why Is The Bottom Of The Periodic Table Separated.
From utedzz.blogspot.com
Labeled Separated Group Periodic Table Groups Periodic Table Timeline Why Is The Bottom Of The Periodic Table Separated The two rows of elements present at the bottom of the periodic table are called the 4f series or lanthanoids and 5f or actanoids. These rows contain elements in the lanthanoid. The reason why lanthanides and actinides are located at the bottom of the periodical table is because of their properties and in the block in which electrons fill up.. Why Is The Bottom Of The Periodic Table Separated.