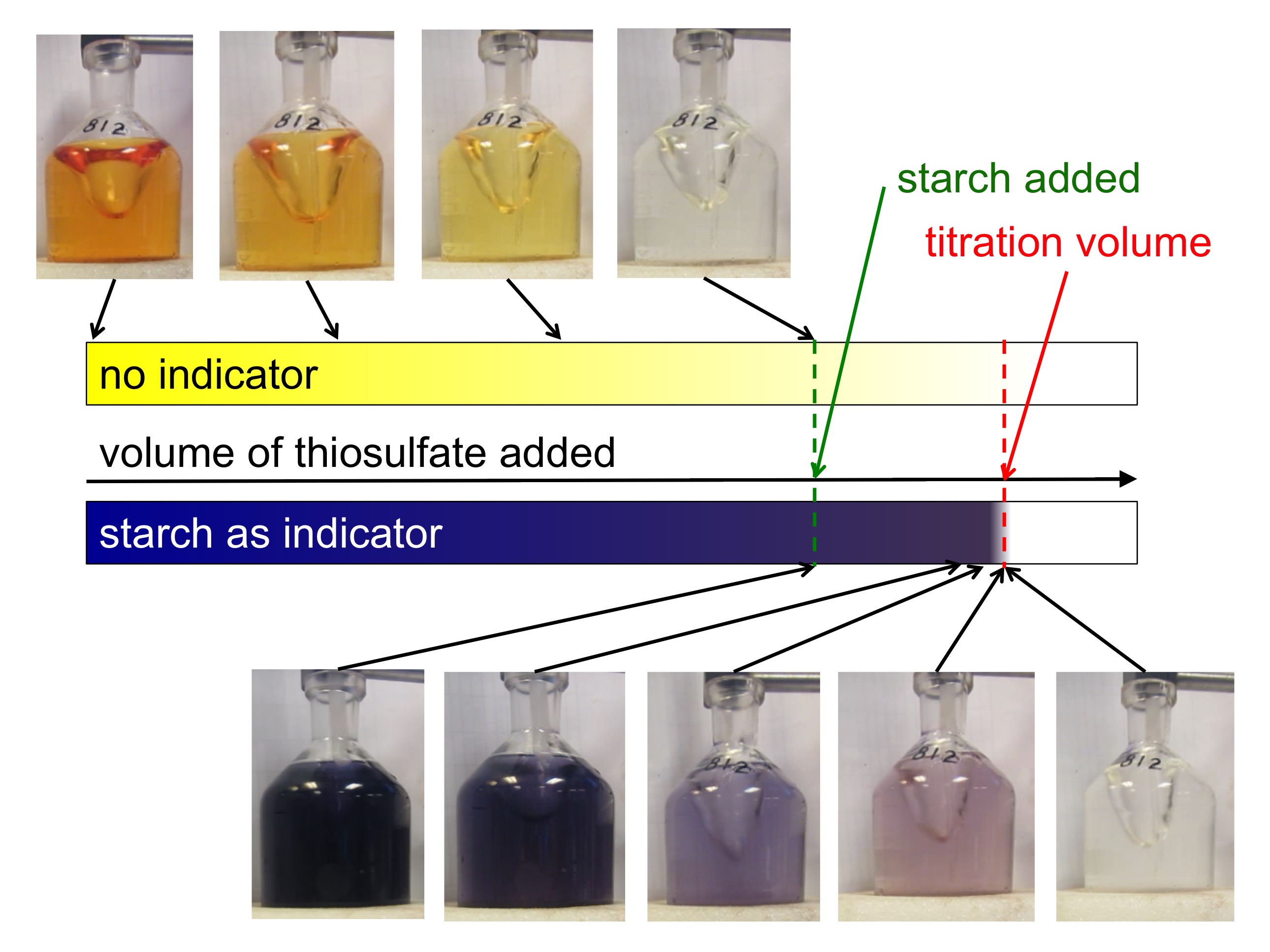
How Does Indicator Affect Titration . Both indicators change colour over a specific ph range. If a large amount of indicator is. Indicators are substances that change colour when they are added to acidic or alkaline solutions. In many titrations, you use a chemical called an indicator, which lets you know when the titration finishes. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. These traits are desirable so only a small amount of an indicator is needed. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. When choosing the appropriate indicator, the ph of the equivalence point is very. Measuring a known volume (usually 20 or 25 cm 3) of one of the solutions with a volumetric or graduated pipette and placing it into a conical flask; The steps in a titration are: In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. A useful indicator has a strong color that changes quickly near its pka.
from mirjamglessmer.com
In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. Measuring a known volume (usually 20 or 25 cm 3) of one of the solutions with a volumetric or graduated pipette and placing it into a conical flask; A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. A useful indicator has a strong color that changes quickly near its pka. In many titrations, you use a chemical called an indicator, which lets you know when the titration finishes. Indicators are substances that change colour when they are added to acidic or alkaline solutions. When choosing the appropriate indicator, the ph of the equivalence point is very. The steps in a titration are: Both indicators change colour over a specific ph range. These traits are desirable so only a small amount of an indicator is needed.
Winkler titration Archives Adventures in Oceanography and Teaching
How Does Indicator Affect Titration If a large amount of indicator is. Measuring a known volume (usually 20 or 25 cm 3) of one of the solutions with a volumetric or graduated pipette and placing it into a conical flask; Both indicators change colour over a specific ph range. If a large amount of indicator is. In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. A useful indicator has a strong color that changes quickly near its pka. When choosing the appropriate indicator, the ph of the equivalence point is very. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. The steps in a titration are: Indicators are substances that change colour when they are added to acidic or alkaline solutions. In many titrations, you use a chemical called an indicator, which lets you know when the titration finishes. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. These traits are desirable so only a small amount of an indicator is needed.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre How Does Indicator Affect Titration The two most common indicators that are used in titrations are methyl orange and phenolphthalein. In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. When choosing the appropriate indicator, the ph of the equivalence point is very. These traits are desirable so only a small amount of an indicator is. How Does Indicator Affect Titration.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii How Does Indicator Affect Titration Indicators are substances that change colour when they are added to acidic or alkaline solutions. When choosing the appropriate indicator, the ph of the equivalence point is very. In many titrations, you use a chemical called an indicator, which lets you know when the titration finishes. Both indicators change colour over a specific ph range. A titration is a volumetric. How Does Indicator Affect Titration.
From general.chemistrysteps.com
Titration of a Weak Acid by a Strong Base Chemistry Steps How Does Indicator Affect Titration If a large amount of indicator is. When choosing the appropriate indicator, the ph of the equivalence point is very. In many titrations, you use a chemical called an indicator, which lets you know when the titration finishes. In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. A titration is. How Does Indicator Affect Titration.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators How Does Indicator Affect Titration These traits are desirable so only a small amount of an indicator is needed. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. The steps in a titration are: In many titrations, you use a chemical called. How Does Indicator Affect Titration.
From mirjamglessmer.com
Winkler titration Archives Adventures in Oceanography and Teaching How Does Indicator Affect Titration In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. In many titrations, you use a chemical called an. How Does Indicator Affect Titration.
From franco-krussell.blogspot.com
How to Determine Which Indicator to Use for Titration How Does Indicator Affect Titration Indicators are substances that change colour when they are added to acidic or alkaline solutions. The steps in a titration are: If a large amount of indicator is. In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. A useful indicator has a strong color that changes quickly near its pka.. How Does Indicator Affect Titration.
From www.slideserve.com
PPT Volumetric ANALYSIS/TITRATION PowerPoint Presentation, free How Does Indicator Affect Titration When choosing the appropriate indicator, the ph of the equivalence point is very. In many titrations, you use a chemical called an indicator, which lets you know when the titration finishes. In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. These traits are desirable so only a small amount of. How Does Indicator Affect Titration.
From mungfali.com
Acid Base Titration Indicator How Does Indicator Affect Titration A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. When choosing the appropriate indicator, the ph of the equivalence point is very. In many titrations, you use a chemical called an indicator, which lets you know when. How Does Indicator Affect Titration.
From theedge.com.hk
Chemistry How To Titration The Edge How Does Indicator Affect Titration Measuring a known volume (usually 20 or 25 cm 3) of one of the solutions with a volumetric or graduated pipette and placing it into a conical flask; These traits are desirable so only a small amount of an indicator is needed. If a large amount of indicator is. In many titrations, you use a chemical called an indicator, which. How Does Indicator Affect Titration.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic How Does Indicator Affect Titration In many titrations, you use a chemical called an indicator, which lets you know when the titration finishes. When choosing the appropriate indicator, the ph of the equivalence point is very. Indicators are substances that change colour when they are added to acidic or alkaline solutions. A titration is a volumetric technique in which a solution of one reactant (the. How Does Indicator Affect Titration.
From www.vrogue.co
What Is Titration And How Does It Work vrogue.co How Does Indicator Affect Titration These traits are desirable so only a small amount of an indicator is needed. When choosing the appropriate indicator, the ph of the equivalence point is very. Measuring a known volume (usually 20 or 25 cm 3) of one of the solutions with a volumetric or graduated pipette and placing it into a conical flask; The steps in a titration. How Does Indicator Affect Titration.
From www.youtube.com
Titration of a monoprotic strong acid (HCl) and monoprotic strong base How Does Indicator Affect Titration These traits are desirable so only a small amount of an indicator is needed. If a large amount of indicator is. Measuring a known volume (usually 20 or 25 cm 3) of one of the solutions with a volumetric or graduated pipette and placing it into a conical flask; The steps in a titration are: Indicators are substances that change. How Does Indicator Affect Titration.
From www.slideserve.com
PPT Titrations PowerPoint Presentation, free download ID5572517 How Does Indicator Affect Titration The steps in a titration are: Indicators are substances that change colour when they are added to acidic or alkaline solutions. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. If a large amount of. How Does Indicator Affect Titration.
From www.reagent.co.uk
How is Titration Used in the Pharmaceutical Industry? How Does Indicator Affect Titration These traits are desirable so only a small amount of an indicator is needed. Indicators are substances that change colour when they are added to acidic or alkaline solutions. Both indicators change colour over a specific ph range. A useful indicator has a strong color that changes quickly near its pka. The steps in a titration are: If a large. How Does Indicator Affect Titration.
From wisc.pb.unizin.org
D30.1 AcidBase Indicators Chemistry 109 Fall 2021 How Does Indicator Affect Titration A useful indicator has a strong color that changes quickly near its pka. In many titrations, you use a chemical called an indicator, which lets you know when the titration finishes. When choosing the appropriate indicator, the ph of the equivalence point is very. These traits are desirable so only a small amount of an indicator is needed. Measuring a. How Does Indicator Affect Titration.
From studylib.net
The titration of an Indicator (HIn) How Does Indicator Affect Titration A useful indicator has a strong color that changes quickly near its pka. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. A titration is a volumetric technique in which a solution of one reactant. How Does Indicator Affect Titration.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic How Does Indicator Affect Titration The steps in a titration are: A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. Both indicators change colour over a specific ph range. These traits are desirable so only a small amount of an indicator is. How Does Indicator Affect Titration.
From general.chemistrysteps.com
Titration of a Polyprotic Acids Chemistry Steps How Does Indicator Affect Titration When choosing the appropriate indicator, the ph of the equivalence point is very. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Both indicators change colour over a specific ph range. These traits are desirable so only a small amount of an indicator is needed. Measuring a known volume (usually 20 or 25 cm. How Does Indicator Affect Titration.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts How Does Indicator Affect Titration The steps in a titration are: These traits are desirable so only a small amount of an indicator is needed. When choosing the appropriate indicator, the ph of the equivalence point is very. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. A useful indicator has a strong color that changes quickly near its. How Does Indicator Affect Titration.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre How Does Indicator Affect Titration A useful indicator has a strong color that changes quickly near its pka. These traits are desirable so only a small amount of an indicator is needed. In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. When choosing the appropriate indicator, the ph of the equivalence point is very. A. How Does Indicator Affect Titration.
From chem.libretexts.org
Chapter 16.5 AcidBase Titrations Chemistry LibreTexts How Does Indicator Affect Titration If a large amount of indicator is. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. In many titrations, you use a chemical called an indicator, which lets you know when the titration finishes. Measuring. How Does Indicator Affect Titration.
From ar.inspiredpencil.com
Titration Experiment Using Phenolphthalein How Does Indicator Affect Titration In many titrations, you use a chemical called an indicator, which lets you know when the titration finishes. The steps in a titration are: A useful indicator has a strong color that changes quickly near its pka. Both indicators change colour over a specific ph range. The two most common indicators that are used in titrations are methyl orange and. How Does Indicator Affect Titration.
From www.youtube.com
HOW TO DO DOUBLE INDICATOR TITRATION EXPERIMENTS.Chemistry YouTube How Does Indicator Affect Titration If a large amount of indicator is. Indicators are substances that change colour when they are added to acidic or alkaline solutions. A useful indicator has a strong color that changes quickly near its pka. Both indicators change colour over a specific ph range. In a titration, you determine an unknown concentration of a sample by adding a second reactant. How Does Indicator Affect Titration.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download ID How Does Indicator Affect Titration In many titrations, you use a chemical called an indicator, which lets you know when the titration finishes. In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. Indicators are substances that change colour when they are added to acidic or alkaline solutions. When choosing the appropriate indicator, the ph of. How Does Indicator Affect Titration.
From chrominfo.blogspot.com
Chrominfo Indicators of complexometric titration How Does Indicator Affect Titration If a large amount of indicator is. A useful indicator has a strong color that changes quickly near its pka. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. In many titrations, you use a chemical called. How Does Indicator Affect Titration.
From www.science-revision.co.uk
Titrations How Does Indicator Affect Titration Indicators are substances that change colour when they are added to acidic or alkaline solutions. In many titrations, you use a chemical called an indicator, which lets you know when the titration finishes. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until. How Does Indicator Affect Titration.
From www.slideserve.com
PPT Ch 6 Precipitation Titrations, Sec 65 and 66 PowerPoint How Does Indicator Affect Titration In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. In many titrations, you use a chemical called an indicator, which lets you know when the titration finishes. Both indicators change colour over a specific ph range. The two most common indicators that are used in titrations are methyl orange and. How Does Indicator Affect Titration.
From owlcation.com
What Is Titration? The 3 Types of Titration Explained Owlcation How Does Indicator Affect Titration In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. The steps in a titration are: A useful indicator has a strong color that changes quickly near its pka. Indicators are substances that change colour when they are added to acidic or alkaline solutions. Both indicators change colour over a specific. How Does Indicator Affect Titration.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts How Does Indicator Affect Titration The steps in a titration are: The two most common indicators that are used in titrations are methyl orange and phenolphthalein. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. Both indicators change colour over a specific. How Does Indicator Affect Titration.
From franco-krussell.blogspot.com
How to Determine Which Indicator to Use for Titration How Does Indicator Affect Titration A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. The steps in a titration are: These traits are desirable so only a small amount of an indicator is needed. When choosing the appropriate indicator, the ph of. How Does Indicator Affect Titration.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators How Does Indicator Affect Titration In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. Indicators are substances that change colour when they are added to acidic or alkaline solutions. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. The steps in a titration are: A useful indicator has a. How Does Indicator Affect Titration.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators How Does Indicator Affect Titration A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. A useful indicator has a strong color that changes quickly near its pka. When choosing the appropriate indicator, the ph of the equivalence point is very. Indicators are. How Does Indicator Affect Titration.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent How Does Indicator Affect Titration In many titrations, you use a chemical called an indicator, which lets you know when the titration finishes. In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second. How Does Indicator Affect Titration.
From jeparatd.blogspot.com
Why Does The Indicator Change Color In Titration Jeparat How Does Indicator Affect Titration Both indicators change colour over a specific ph range. The steps in a titration are: When choosing the appropriate indicator, the ph of the equivalence point is very. These traits are desirable so only a small amount of an indicator is needed. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. A titration is. How Does Indicator Affect Titration.
From chemwiki.ucdavis.edu
Titration of a Weak Base with a Strong Acid Chemwiki How Does Indicator Affect Titration If a large amount of indicator is. In many titrations, you use a chemical called an indicator, which lets you know when the titration finishes. These traits are desirable so only a small amount of an indicator is needed. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of. How Does Indicator Affect Titration.