Standard Reduction Potential Practice Problems . If the voltage of the cell is −0.72 v, what is the standard reduction potential of the v 3+ + 3e − → v half reaction? For the electrochemical cells shown below, write the cell reactions and find \(\mathrm{e^\circ_{cell}}\) using the standard reduction potential table \(\mathrm{cu(s) | cu^{2+}(aq) || ag^+(aq) | ag(s)}\) Standard reduction potential of mg2+! If galvanic cells have a maximum voltage of only a few volts, how is it possible. Mg / mg 2+(aq) // zn2+(aq) / zn e˚ = +1.61 v a. What species is being oxidized and what species is being reduced in a dry cell? A positive standard reduction potential indicates that a species has a greater tendency to gain electrons and be reduced, while a negative. 3pb + 2v 3 + → 3pb 2 + + 2v; A voltaic cell is based on this reaction: Electrochemistry practice problems include questions on balancing redox reactions in acidic and basic solutions, calculating the cell potential, eo / e at standard conditions using the table of standard reduction potentials, calculating the free energy change, δ g based on the cell potential, calculating the cell potential under nonstandard. What is the difference between ecell and e°cell? The cell is set up at standard conditions so the question can be answered using standard potentials.
from www.chegg.com
If galvanic cells have a maximum voltage of only a few volts, how is it possible. Mg / mg 2+(aq) // zn2+(aq) / zn e˚ = +1.61 v a. 3pb + 2v 3 + → 3pb 2 + + 2v; What is the difference between ecell and e°cell? Electrochemistry practice problems include questions on balancing redox reactions in acidic and basic solutions, calculating the cell potential, eo / e at standard conditions using the table of standard reduction potentials, calculating the free energy change, δ g based on the cell potential, calculating the cell potential under nonstandard. For the electrochemical cells shown below, write the cell reactions and find \(\mathrm{e^\circ_{cell}}\) using the standard reduction potential table \(\mathrm{cu(s) | cu^{2+}(aq) || ag^+(aq) | ag(s)}\) What species is being oxidized and what species is being reduced in a dry cell? If the voltage of the cell is −0.72 v, what is the standard reduction potential of the v 3+ + 3e − → v half reaction? The cell is set up at standard conditions so the question can be answered using standard potentials. Standard reduction potential of mg2+!
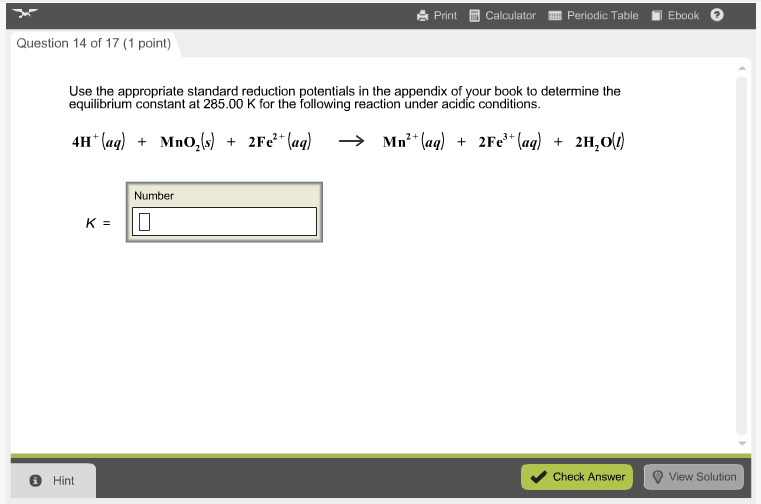
Solved Use the appropriate standard reduction potentials in
Standard Reduction Potential Practice Problems The cell is set up at standard conditions so the question can be answered using standard potentials. If the voltage of the cell is −0.72 v, what is the standard reduction potential of the v 3+ + 3e − → v half reaction? Standard reduction potential of mg2+! A voltaic cell is based on this reaction: What species is being oxidized and what species is being reduced in a dry cell? Electrochemistry practice problems include questions on balancing redox reactions in acidic and basic solutions, calculating the cell potential, eo / e at standard conditions using the table of standard reduction potentials, calculating the free energy change, δ g based on the cell potential, calculating the cell potential under nonstandard. For the electrochemical cells shown below, write the cell reactions and find \(\mathrm{e^\circ_{cell}}\) using the standard reduction potential table \(\mathrm{cu(s) | cu^{2+}(aq) || ag^+(aq) | ag(s)}\) Mg / mg 2+(aq) // zn2+(aq) / zn e˚ = +1.61 v a. 3pb + 2v 3 + → 3pb 2 + + 2v; A positive standard reduction potential indicates that a species has a greater tendency to gain electrons and be reduced, while a negative. The cell is set up at standard conditions so the question can be answered using standard potentials. What is the difference between ecell and e°cell? If galvanic cells have a maximum voltage of only a few volts, how is it possible.
From www.coursehero.com
[Solved] Table 1 Some standard reduction potentials (298 K) Half Standard Reduction Potential Practice Problems A positive standard reduction potential indicates that a species has a greater tendency to gain electrons and be reduced, while a negative. A voltaic cell is based on this reaction: Mg / mg 2+(aq) // zn2+(aq) / zn e˚ = +1.61 v a. What is the difference between ecell and e°cell? Electrochemistry practice problems include questions on balancing redox reactions. Standard Reduction Potential Practice Problems.
From www.pveducation.org
Standard Potential PVEducation Standard Reduction Potential Practice Problems A positive standard reduction potential indicates that a species has a greater tendency to gain electrons and be reduced, while a negative. Electrochemistry practice problems include questions on balancing redox reactions in acidic and basic solutions, calculating the cell potential, eo / e at standard conditions using the table of standard reduction potentials, calculating the free energy change, δ g. Standard Reduction Potential Practice Problems.
From www.chegg.com
Solved The standard reduction potentials for the following Standard Reduction Potential Practice Problems Mg / mg 2+(aq) // zn2+(aq) / zn e˚ = +1.61 v a. For the electrochemical cells shown below, write the cell reactions and find \(\mathrm{e^\circ_{cell}}\) using the standard reduction potential table \(\mathrm{cu(s) | cu^{2+}(aq) || ag^+(aq) | ag(s)}\) 3pb + 2v 3 + → 3pb 2 + + 2v; A voltaic cell is based on this reaction: If galvanic. Standard Reduction Potential Practice Problems.
From www.chegg.com
Solved Standard (reduction) potentials are given below for Standard Reduction Potential Practice Problems For the electrochemical cells shown below, write the cell reactions and find \(\mathrm{e^\circ_{cell}}\) using the standard reduction potential table \(\mathrm{cu(s) | cu^{2+}(aq) || ag^+(aq) | ag(s)}\) Mg / mg 2+(aq) // zn2+(aq) / zn e˚ = +1.61 v a. What is the difference between ecell and e°cell? A positive standard reduction potential indicates that a species has a greater tendency. Standard Reduction Potential Practice Problems.
From www.chegg.com
Solved Using standard reduction potentials below, calculate Standard Reduction Potential Practice Problems If galvanic cells have a maximum voltage of only a few volts, how is it possible. What is the difference between ecell and e°cell? If the voltage of the cell is −0.72 v, what is the standard reduction potential of the v 3+ + 3e − → v half reaction? What species is being oxidized and what species is being. Standard Reduction Potential Practice Problems.
From f15.beauty
Standard Reduction Potential Table Standard Reduction Potential Practice Problems The cell is set up at standard conditions so the question can be answered using standard potentials. For the electrochemical cells shown below, write the cell reactions and find \(\mathrm{e^\circ_{cell}}\) using the standard reduction potential table \(\mathrm{cu(s) | cu^{2+}(aq) || ag^+(aq) | ag(s)}\) If the voltage of the cell is −0.72 v, what is the standard reduction potential of the. Standard Reduction Potential Practice Problems.
From www.chegg.com
Solved Use standard reduction potentials to calculate the Standard Reduction Potential Practice Problems The cell is set up at standard conditions so the question can be answered using standard potentials. A voltaic cell is based on this reaction: If the voltage of the cell is −0.72 v, what is the standard reduction potential of the v 3+ + 3e − → v half reaction? 3pb + 2v 3 + → 3pb 2 +. Standard Reduction Potential Practice Problems.
From www.chegg.com
Solved Use the appropriate standard reduction potentials in Standard Reduction Potential Practice Problems A positive standard reduction potential indicates that a species has a greater tendency to gain electrons and be reduced, while a negative. What species is being oxidized and what species is being reduced in a dry cell? A voltaic cell is based on this reaction: Standard reduction potential of mg2+! 3pb + 2v 3 + → 3pb 2 + +. Standard Reduction Potential Practice Problems.
From www.chegg.com
Solved Use the standard reduction potentials given below to Standard Reduction Potential Practice Problems A voltaic cell is based on this reaction: If galvanic cells have a maximum voltage of only a few volts, how is it possible. If the voltage of the cell is −0.72 v, what is the standard reduction potential of the v 3+ + 3e − → v half reaction? 3pb + 2v 3 + → 3pb 2 + +. Standard Reduction Potential Practice Problems.
From www.chegg.com
Solved Use standard reduction potentials to calculate the Standard Reduction Potential Practice Problems 3pb + 2v 3 + → 3pb 2 + + 2v; What is the difference between ecell and e°cell? The cell is set up at standard conditions so the question can be answered using standard potentials. Standard reduction potential of mg2+! Mg / mg 2+(aq) // zn2+(aq) / zn e˚ = +1.61 v a. A voltaic cell is based on. Standard Reduction Potential Practice Problems.
From www.numerade.com
SOLVED The standard reduction potentials of the following half Standard Reduction Potential Practice Problems Electrochemistry practice problems include questions on balancing redox reactions in acidic and basic solutions, calculating the cell potential, eo / e at standard conditions using the table of standard reduction potentials, calculating the free energy change, δ g based on the cell potential, calculating the cell potential under nonstandard. For the electrochemical cells shown below, write the cell reactions and. Standard Reduction Potential Practice Problems.
From www.chegg.com
Solved Standard reduction potentials Use the table of Standard Reduction Potential Practice Problems 3pb + 2v 3 + → 3pb 2 + + 2v; Standard reduction potential of mg2+! A voltaic cell is based on this reaction: A positive standard reduction potential indicates that a species has a greater tendency to gain electrons and be reduced, while a negative. Electrochemistry practice problems include questions on balancing redox reactions in acidic and basic solutions,. Standard Reduction Potential Practice Problems.
From www.chegg.com
Solved 4. (a) Use the standard reduction potentials at 25° C Standard Reduction Potential Practice Problems If galvanic cells have a maximum voltage of only a few volts, how is it possible. What is the difference between ecell and e°cell? Mg / mg 2+(aq) // zn2+(aq) / zn e˚ = +1.61 v a. A positive standard reduction potential indicates that a species has a greater tendency to gain electrons and be reduced, while a negative. For. Standard Reduction Potential Practice Problems.
From www.chegg.com
Solved 4. (a) Use The Standard Reduction Potentials At 25... Standard Reduction Potential Practice Problems A voltaic cell is based on this reaction: What species is being oxidized and what species is being reduced in a dry cell? Mg / mg 2+(aq) // zn2+(aq) / zn e˚ = +1.61 v a. For the electrochemical cells shown below, write the cell reactions and find \(\mathrm{e^\circ_{cell}}\) using the standard reduction potential table \(\mathrm{cu(s) | cu^{2+}(aq) || ag^+(aq). Standard Reduction Potential Practice Problems.
From www.numerade.com
SOLVEDExample 4 Using standard reduction potentials determine which of Standard Reduction Potential Practice Problems Electrochemistry practice problems include questions on balancing redox reactions in acidic and basic solutions, calculating the cell potential, eo / e at standard conditions using the table of standard reduction potentials, calculating the free energy change, δ g based on the cell potential, calculating the cell potential under nonstandard. Mg / mg 2+(aq) // zn2+(aq) / zn e˚ = +1.61. Standard Reduction Potential Practice Problems.
From www.chegg.com
Solved Given these standard reduction potentials at 25 Standard Reduction Potential Practice Problems Standard reduction potential of mg2+! Electrochemistry practice problems include questions on balancing redox reactions in acidic and basic solutions, calculating the cell potential, eo / e at standard conditions using the table of standard reduction potentials, calculating the free energy change, δ g based on the cell potential, calculating the cell potential under nonstandard. What is the difference between ecell. Standard Reduction Potential Practice Problems.
From solvedlib.com
Use standard reduction potentials to calculate the eq… SolvedLib Standard Reduction Potential Practice Problems Standard reduction potential of mg2+! What is the difference between ecell and e°cell? Mg / mg 2+(aq) // zn2+(aq) / zn e˚ = +1.61 v a. A positive standard reduction potential indicates that a species has a greater tendency to gain electrons and be reduced, while a negative. If the voltage of the cell is −0.72 v, what is the. Standard Reduction Potential Practice Problems.
From www.chegg.com
Solved using the standard reduction potentials Determine if Standard Reduction Potential Practice Problems Electrochemistry practice problems include questions on balancing redox reactions in acidic and basic solutions, calculating the cell potential, eo / e at standard conditions using the table of standard reduction potentials, calculating the free energy change, δ g based on the cell potential, calculating the cell potential under nonstandard. What is the difference between ecell and e°cell? A positive standard. Standard Reduction Potential Practice Problems.
From www.coursehero.com
[Solved] Consider the following standard reduction potentials in acid Standard Reduction Potential Practice Problems The cell is set up at standard conditions so the question can be answered using standard potentials. Electrochemistry practice problems include questions on balancing redox reactions in acidic and basic solutions, calculating the cell potential, eo / e at standard conditions using the table of standard reduction potentials, calculating the free energy change, δ g based on the cell potential,. Standard Reduction Potential Practice Problems.
From www.flinnsci.ca
Standard Reduction Potential Charts for Chemistry Standard Reduction Potential Practice Problems If galvanic cells have a maximum voltage of only a few volts, how is it possible. A positive standard reduction potential indicates that a species has a greater tendency to gain electrons and be reduced, while a negative. If the voltage of the cell is −0.72 v, what is the standard reduction potential of the v 3+ + 3e −. Standard Reduction Potential Practice Problems.
From www.chegg.com
Solved Use standard reduction potentials to calculate the Standard Reduction Potential Practice Problems Electrochemistry practice problems include questions on balancing redox reactions in acidic and basic solutions, calculating the cell potential, eo / e at standard conditions using the table of standard reduction potentials, calculating the free energy change, δ g based on the cell potential, calculating the cell potential under nonstandard. For the electrochemical cells shown below, write the cell reactions and. Standard Reduction Potential Practice Problems.
From www.coursehero.com
[Solved] 1) Use standard reduction potentials to calculate the standard Standard Reduction Potential Practice Problems A positive standard reduction potential indicates that a species has a greater tendency to gain electrons and be reduced, while a negative. The cell is set up at standard conditions so the question can be answered using standard potentials. 3pb + 2v 3 + → 3pb 2 + + 2v; Electrochemistry practice problems include questions on balancing redox reactions in. Standard Reduction Potential Practice Problems.
From www.coursehero.com
[Solved] Consider the following standard reduction potentials, A13+(aq Standard Reduction Potential Practice Problems Mg / mg 2+(aq) // zn2+(aq) / zn e˚ = +1.61 v a. If galvanic cells have a maximum voltage of only a few volts, how is it possible. Standard reduction potential of mg2+! 3pb + 2v 3 + → 3pb 2 + + 2v; For the electrochemical cells shown below, write the cell reactions and find \(\mathrm{e^\circ_{cell}}\) using the. Standard Reduction Potential Practice Problems.
From www.solutionspile.com
[Solved] Use the standard reduction potentials given bel Standard Reduction Potential Practice Problems Standard reduction potential of mg2+! A positive standard reduction potential indicates that a species has a greater tendency to gain electrons and be reduced, while a negative. What is the difference between ecell and e°cell? If the voltage of the cell is −0.72 v, what is the standard reduction potential of the v 3+ + 3e − → v half. Standard Reduction Potential Practice Problems.
From www.studocu.com
Chapter19Worksheet Chapter 19 Worksheet (You will need to look up Standard Reduction Potential Practice Problems What is the difference between ecell and e°cell? A voltaic cell is based on this reaction: If galvanic cells have a maximum voltage of only a few volts, how is it possible. For the electrochemical cells shown below, write the cell reactions and find \(\mathrm{e^\circ_{cell}}\) using the standard reduction potential table \(\mathrm{cu(s) | cu^{2+}(aq) || ag^+(aq) | ag(s)}\) Electrochemistry practice. Standard Reduction Potential Practice Problems.
From www.chegg.com
Solved Use standard reduction potentials to calculate the Standard Reduction Potential Practice Problems A positive standard reduction potential indicates that a species has a greater tendency to gain electrons and be reduced, while a negative. 3pb + 2v 3 + → 3pb 2 + + 2v; What species is being oxidized and what species is being reduced in a dry cell? A voltaic cell is based on this reaction: For the electrochemical cells. Standard Reduction Potential Practice Problems.
From www.chegg.com
Solved Use standard reduction potentials to calculate the Standard Reduction Potential Practice Problems For the electrochemical cells shown below, write the cell reactions and find \(\mathrm{e^\circ_{cell}}\) using the standard reduction potential table \(\mathrm{cu(s) | cu^{2+}(aq) || ag^+(aq) | ag(s)}\) 3pb + 2v 3 + → 3pb 2 + + 2v; What is the difference between ecell and e°cell? What species is being oxidized and what species is being reduced in a dry cell?. Standard Reduction Potential Practice Problems.
From www.numerade.com
SOLVED Table 2. Table of Selected Standard Reduction Potentials at 25Â Standard Reduction Potential Practice Problems For the electrochemical cells shown below, write the cell reactions and find \(\mathrm{e^\circ_{cell}}\) using the standard reduction potential table \(\mathrm{cu(s) | cu^{2+}(aq) || ag^+(aq) | ag(s)}\) What is the difference between ecell and e°cell? 3pb + 2v 3 + → 3pb 2 + + 2v; A voltaic cell is based on this reaction: Standard reduction potential of mg2+! If the. Standard Reduction Potential Practice Problems.
From www.chegg.com
Solved Using standard reduction potentials from the ALEKS Standard Reduction Potential Practice Problems What species is being oxidized and what species is being reduced in a dry cell? 3pb + 2v 3 + → 3pb 2 + + 2v; For the electrochemical cells shown below, write the cell reactions and find \(\mathrm{e^\circ_{cell}}\) using the standard reduction potential table \(\mathrm{cu(s) | cu^{2+}(aq) || ag^+(aq) | ag(s)}\) Standard reduction potential of mg2+! Mg / mg. Standard Reduction Potential Practice Problems.
From www.chegg.com
Solved Using standard reduction potentials from the ALEKS Standard Reduction Potential Practice Problems For the electrochemical cells shown below, write the cell reactions and find \(\mathrm{e^\circ_{cell}}\) using the standard reduction potential table \(\mathrm{cu(s) | cu^{2+}(aq) || ag^+(aq) | ag(s)}\) A voltaic cell is based on this reaction: If galvanic cells have a maximum voltage of only a few volts, how is it possible. A positive standard reduction potential indicates that a species has. Standard Reduction Potential Practice Problems.
From www.chegg.com
Solved Using the standard reduction potentials from the Standard Reduction Potential Practice Problems If galvanic cells have a maximum voltage of only a few volts, how is it possible. If the voltage of the cell is −0.72 v, what is the standard reduction potential of the v 3+ + 3e − → v half reaction? A positive standard reduction potential indicates that a species has a greater tendency to gain electrons and be. Standard Reduction Potential Practice Problems.
From www.chegg.com
Solved Use the table of standard reduction potentials 21. Standard Reduction Potential Practice Problems The cell is set up at standard conditions so the question can be answered using standard potentials. For the electrochemical cells shown below, write the cell reactions and find \(\mathrm{e^\circ_{cell}}\) using the standard reduction potential table \(\mathrm{cu(s) | cu^{2+}(aq) || ag^+(aq) | ag(s)}\) Mg / mg 2+(aq) // zn2+(aq) / zn e˚ = +1.61 v a. Standard reduction potential of. Standard Reduction Potential Practice Problems.
From www.chegg.com
Solved 4.) From the standard reduction potentials (E table Standard Reduction Potential Practice Problems What species is being oxidized and what species is being reduced in a dry cell? What is the difference between ecell and e°cell? For the electrochemical cells shown below, write the cell reactions and find \(\mathrm{e^\circ_{cell}}\) using the standard reduction potential table \(\mathrm{cu(s) | cu^{2+}(aq) || ag^+(aq) | ag(s)}\) The cell is set up at standard conditions so the question. Standard Reduction Potential Practice Problems.
From www.numerade.com
SOLVEDUse standard reduction potentials to calculate the equilibrium Standard Reduction Potential Practice Problems The cell is set up at standard conditions so the question can be answered using standard potentials. If galvanic cells have a maximum voltage of only a few volts, how is it possible. For the electrochemical cells shown below, write the cell reactions and find \(\mathrm{e^\circ_{cell}}\) using the standard reduction potential table \(\mathrm{cu(s) | cu^{2+}(aq) || ag^+(aq) | ag(s)}\) Electrochemistry. Standard Reduction Potential Practice Problems.
From study.com
Quiz & Worksheet Standard Reduction Potentials Standard Reduction Potential Practice Problems Standard reduction potential of mg2+! 3pb + 2v 3 + → 3pb 2 + + 2v; A positive standard reduction potential indicates that a species has a greater tendency to gain electrons and be reduced, while a negative. What is the difference between ecell and e°cell? Electrochemistry practice problems include questions on balancing redox reactions in acidic and basic solutions,. Standard Reduction Potential Practice Problems.