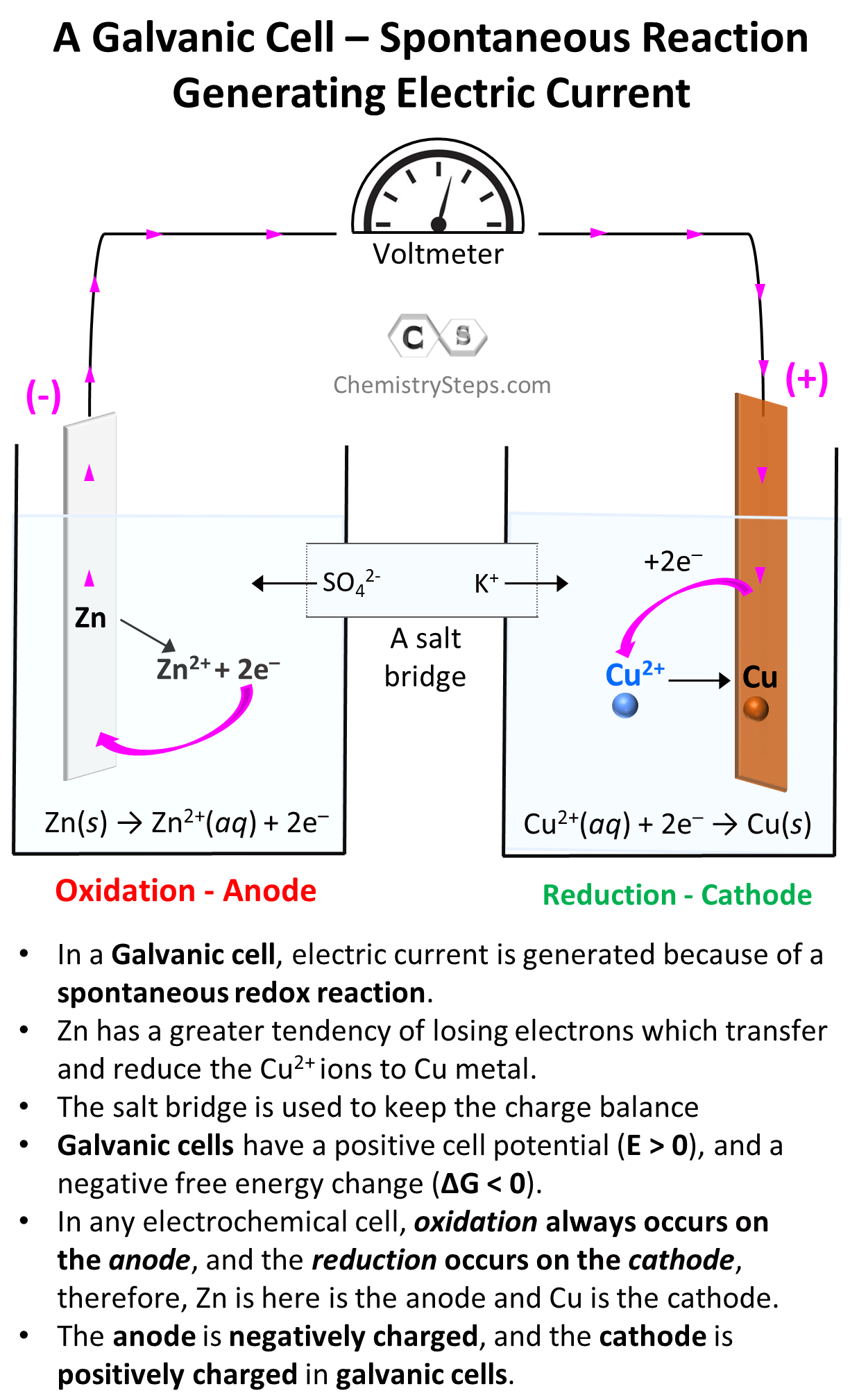
Silver Electrode In Galvanic Cell . At this point, no current flows—that is, no significant movement of. Why is a salt bridge necessary in galvanic. At this point, no current flows—that is, no significant. An external circuit is connected to. A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process.
from general.chemistrysteps.com
At this point, no current flows—that is, no significant movement of. A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. At this point, no current flows—that is, no significant. An external circuit is connected to. Why is a salt bridge necessary in galvanic.
Galvanic Cells Chemistry Steps
Silver Electrode In Galvanic Cell At this point, no current flows—that is, no significant movement of. An external circuit is connected to. Why is a salt bridge necessary in galvanic. A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. At this point, no current flows—that is, no significant. At this point, no current flows—that is, no significant movement of.
From saylordotorg.github.io
Electrochemistry Silver Electrode In Galvanic Cell An external circuit is connected to. At this point, no current flows—that is, no significant. A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. Why is a salt bridge necessary in galvanic. At this point, no current flows—that is, no significant movement of. Silver Electrode In Galvanic Cell.
From chem.libretexts.org
Voltaic Cells Chemistry LibreTexts Silver Electrode In Galvanic Cell At this point, no current flows—that is, no significant movement of. A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. Why is a salt bridge necessary in galvanic. At this point, no current flows—that is, no significant. An external circuit is connected to. Silver Electrode In Galvanic Cell.
From general.chemistrysteps.com
Galvanic Cells Chemistry Steps Silver Electrode In Galvanic Cell At this point, no current flows—that is, no significant. A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. At this point, no current flows—that is, no significant movement of. An external circuit is connected to. Why is a salt bridge necessary in galvanic. Silver Electrode In Galvanic Cell.
From www.youtube.com
galvanic cell animation Zinc + silver YouTube Silver Electrode In Galvanic Cell Why is a salt bridge necessary in galvanic. At this point, no current flows—that is, no significant movement of. A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. An external circuit is connected to. At this point, no current flows—that is, no significant. Silver Electrode In Galvanic Cell.
From www.jove.com
Voltaic/Galvanic Cells Principle, Components, Cell Notation JoVE Silver Electrode In Galvanic Cell A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. Why is a salt bridge necessary in galvanic. An external circuit is connected to. At this point, no current flows—that is, no significant movement of. At this point, no current flows—that is, no significant. Silver Electrode In Galvanic Cell.
From www.dynamicscience.com.au
chemistry galvanic cells Silver Electrode In Galvanic Cell At this point, no current flows—that is, no significant. A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. Why is a salt bridge necessary in galvanic. An external circuit is connected to. At this point, no current flows—that is, no significant movement of. Silver Electrode In Galvanic Cell.
From saylordotorg.github.io
Electrochemistry Silver Electrode In Galvanic Cell At this point, no current flows—that is, no significant movement of. Why is a salt bridge necessary in galvanic. At this point, no current flows—that is, no significant. A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. An external circuit is connected to. Silver Electrode In Galvanic Cell.
From www.pinterest.com
Electrochemical cell Electrochemistry, Chemistry classroom Silver Electrode In Galvanic Cell Why is a salt bridge necessary in galvanic. At this point, no current flows—that is, no significant. At this point, no current flows—that is, no significant movement of. A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. An external circuit is connected to. Silver Electrode In Galvanic Cell.
From chem.libretexts.org
Chapter 19.7 Electrolysis Chemistry LibreTexts Silver Electrode In Galvanic Cell At this point, no current flows—that is, no significant. An external circuit is connected to. At this point, no current flows—that is, no significant movement of. Why is a salt bridge necessary in galvanic. A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. Silver Electrode In Galvanic Cell.
From www.numerade.com
Consider a galvanic cell made from electrodes and solutions of silver Silver Electrode In Galvanic Cell An external circuit is connected to. Why is a salt bridge necessary in galvanic. A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. At this point, no current flows—that is, no significant movement of. At this point, no current flows—that is, no significant. Silver Electrode In Galvanic Cell.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working Silver Electrode In Galvanic Cell An external circuit is connected to. Why is a salt bridge necessary in galvanic. A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. At this point, no current flows—that is, no significant. At this point, no current flows—that is, no significant movement of. Silver Electrode In Galvanic Cell.
From ar.inspiredpencil.com
Copper Silver Galvanic Cell Silver Electrode In Galvanic Cell At this point, no current flows—that is, no significant. A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. At this point, no current flows—that is, no significant movement of. Why is a salt bridge necessary in galvanic. An external circuit is connected to. Silver Electrode In Galvanic Cell.
From favpng.com
Electrolytic Cell Electrolysis Silver Nitrate Galvanic Cell, PNG Silver Electrode In Galvanic Cell An external circuit is connected to. A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. At this point, no current flows—that is, no significant movement of. Why is a salt bridge necessary in galvanic. At this point, no current flows—that is, no significant. Silver Electrode In Galvanic Cell.
From courses.lumenlearning.com
Galvanic Cells Chemistry Silver Electrode In Galvanic Cell At this point, no current flows—that is, no significant movement of. A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. An external circuit is connected to. Why is a salt bridge necessary in galvanic. At this point, no current flows—that is, no significant. Silver Electrode In Galvanic Cell.
From socratic.org
Two halfcells in a galvanic cell consist of one iron (Fe(s)) electrode Silver Electrode In Galvanic Cell An external circuit is connected to. At this point, no current flows—that is, no significant movement of. Why is a salt bridge necessary in galvanic. A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. At this point, no current flows—that is, no significant. Silver Electrode In Galvanic Cell.
From philschatz.com
Electrolysis · Chemistry Silver Electrode In Galvanic Cell An external circuit is connected to. A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. At this point, no current flows—that is, no significant. At this point, no current flows—that is, no significant movement of. Why is a salt bridge necessary in galvanic. Silver Electrode In Galvanic Cell.
From www.numerade.com
SOLVED Question 19 points) A galvanic cell is made using a silver Silver Electrode In Galvanic Cell Why is a salt bridge necessary in galvanic. An external circuit is connected to. A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. At this point, no current flows—that is, no significant. At this point, no current flows—that is, no significant movement of. Silver Electrode In Galvanic Cell.
From www.numerade.com
SOLVED A galvanic cell is made up of a copper electrode in a 1.0 M Silver Electrode In Galvanic Cell At this point, no current flows—that is, no significant. A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. At this point, no current flows—that is, no significant movement of. An external circuit is connected to. Why is a salt bridge necessary in galvanic. Silver Electrode In Galvanic Cell.
From www.numerade.com
SOLVED Consider a galvanic cell consisting of an iron electrode in Silver Electrode In Galvanic Cell An external circuit is connected to. A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. Why is a salt bridge necessary in galvanic. At this point, no current flows—that is, no significant. At this point, no current flows—that is, no significant movement of. Silver Electrode In Galvanic Cell.
From rohan-has-andersen.blogspot.com
How to Construct a Galvanic Cell RohanhasAndersen Silver Electrode In Galvanic Cell An external circuit is connected to. Why is a salt bridge necessary in galvanic. At this point, no current flows—that is, no significant. At this point, no current flows—that is, no significant movement of. A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. Silver Electrode In Galvanic Cell.
From glossary.periodni.com
Galvanic Chemistry Dictionary & Glossary Silver Electrode In Galvanic Cell Why is a salt bridge necessary in galvanic. An external circuit is connected to. A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. At this point, no current flows—that is, no significant. At this point, no current flows—that is, no significant movement of. Silver Electrode In Galvanic Cell.
From www.numerade.com
A galvanic cell is prepared with tin and silver electrodes in contact Silver Electrode In Galvanic Cell At this point, no current flows—that is, no significant movement of. At this point, no current flows—that is, no significant. An external circuit is connected to. A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. Why is a salt bridge necessary in galvanic. Silver Electrode In Galvanic Cell.
From www.differencebetween.com
Difference Between Galvanic Cell and Concentration Cell Compare the Silver Electrode In Galvanic Cell An external circuit is connected to. Why is a salt bridge necessary in galvanic. A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. At this point, no current flows—that is, no significant. At this point, no current flows—that is, no significant movement of. Silver Electrode In Galvanic Cell.
From courses.lumenlearning.com
Galvanic Cells Chemistry Atoms First Silver Electrode In Galvanic Cell A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. An external circuit is connected to. At this point, no current flows—that is, no significant. Why is a salt bridge necessary in galvanic. At this point, no current flows—that is, no significant movement of. Silver Electrode In Galvanic Cell.
From mallory-blogmercer.blogspot.com
How to Construct a Galvanic Cell Silver Electrode In Galvanic Cell A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. An external circuit is connected to. At this point, no current flows—that is, no significant. Why is a salt bridge necessary in galvanic. At this point, no current flows—that is, no significant movement of. Silver Electrode In Galvanic Cell.
From mavink.com
Galvanic Cell Labeled Silver Electrode In Galvanic Cell At this point, no current flows—that is, no significant. Why is a salt bridge necessary in galvanic. A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. At this point, no current flows—that is, no significant movement of. An external circuit is connected to. Silver Electrode In Galvanic Cell.
From www.geeksforgeeks.org
Galvanic Cell Definition, Construction, Working Principle Silver Electrode In Galvanic Cell At this point, no current flows—that is, no significant. A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. At this point, no current flows—that is, no significant movement of. Why is a salt bridge necessary in galvanic. An external circuit is connected to. Silver Electrode In Galvanic Cell.
From courses.lumenlearning.com
Standard Reduction Potentials Chemistry Silver Electrode In Galvanic Cell Why is a salt bridge necessary in galvanic. At this point, no current flows—that is, no significant movement of. An external circuit is connected to. A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. At this point, no current flows—that is, no significant. Silver Electrode In Galvanic Cell.
From studyfullpapst.z21.web.core.windows.net
Consider The Following Electrochemical Cell Silver Electrode In Galvanic Cell Why is a salt bridge necessary in galvanic. At this point, no current flows—that is, no significant movement of. At this point, no current flows—that is, no significant. An external circuit is connected to. A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. Silver Electrode In Galvanic Cell.
From enginelistmathew.z13.web.core.windows.net
Cathode In Electrochemical Cell Silver Electrode In Galvanic Cell An external circuit is connected to. A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. At this point, no current flows—that is, no significant movement of. At this point, no current flows—that is, no significant. Why is a salt bridge necessary in galvanic. Silver Electrode In Galvanic Cell.
From boisestate.pressbooks.pub
17.3 Standard Reduction Potentials General Chemistry 1 & 2 Silver Electrode In Galvanic Cell At this point, no current flows—that is, no significant. A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. Why is a salt bridge necessary in galvanic. At this point, no current flows—that is, no significant movement of. An external circuit is connected to. Silver Electrode In Galvanic Cell.
From chem.libretexts.org
11.1 Galvanic Cells Chemistry LibreTexts Silver Electrode In Galvanic Cell At this point, no current flows—that is, no significant movement of. At this point, no current flows—that is, no significant. A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. Why is a salt bridge necessary in galvanic. An external circuit is connected to. Silver Electrode In Galvanic Cell.
From www.slideserve.com
PPT Chapter Twenty PowerPoint Presentation, free download ID5368420 Silver Electrode In Galvanic Cell An external circuit is connected to. Why is a salt bridge necessary in galvanic. At this point, no current flows—that is, no significant movement of. A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. At this point, no current flows—that is, no significant. Silver Electrode In Galvanic Cell.
From www.slideserve.com
PPT Galvanic Cells PowerPoint Presentation, free download ID3222668 Silver Electrode In Galvanic Cell At this point, no current flows—that is, no significant movement of. At this point, no current flows—that is, no significant. A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. Why is a salt bridge necessary in galvanic. An external circuit is connected to. Silver Electrode In Galvanic Cell.
From diagramlibrarypern.z21.web.core.windows.net
Cathode In Electrochemical Cell Silver Electrode In Galvanic Cell At this point, no current flows—that is, no significant movement of. Why is a salt bridge necessary in galvanic. A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. An external circuit is connected to. At this point, no current flows—that is, no significant. Silver Electrode In Galvanic Cell.