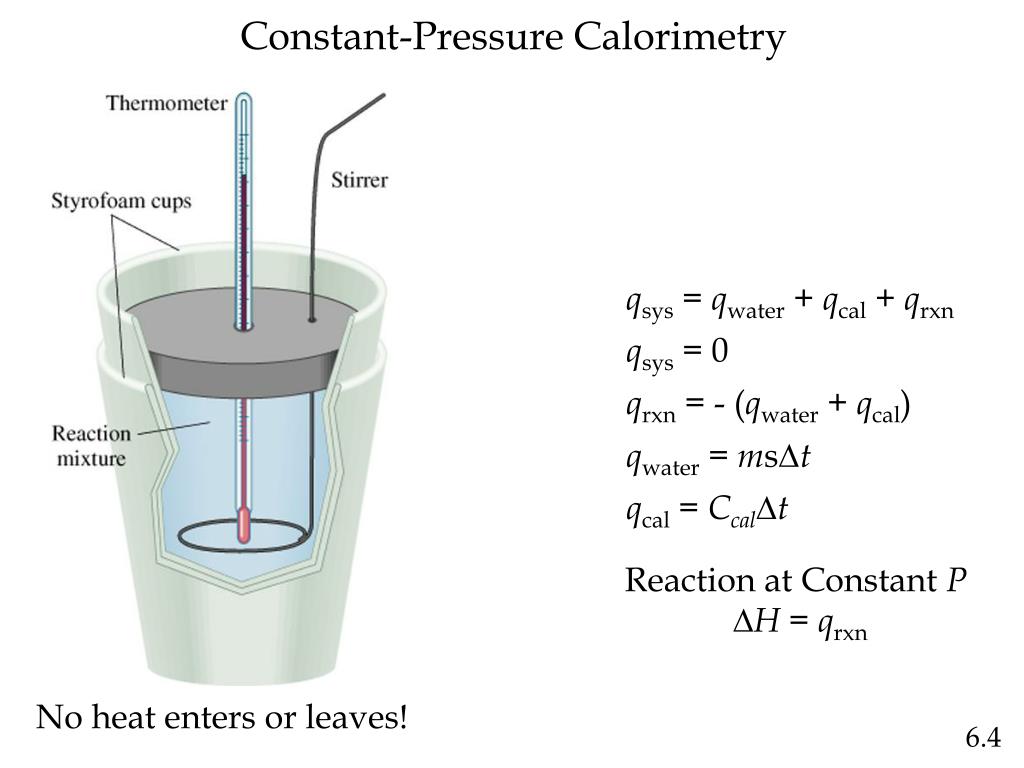
Constant-Pressure Calorimeter Definition . A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. In that case, the gaseous pressure above the solution remains constant, and. For example, when an exothermic. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). The calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow.
from www.slideserve.com
In that case, the gaseous pressure above the solution remains constant, and. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). The calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow.
PPT Chapter 5 PowerPoint Presentation, free download ID1549939
Constant-Pressure Calorimeter Definition Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). The calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. For example, when an exothermic. In that case, the gaseous pressure above the solution remains constant, and. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.
From www.slideserve.com
PPT Heating Curves PowerPoint Presentation, free download ID4576403 Constant-Pressure Calorimeter Definition For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). The calorimeters described are designed to operate at constant (atmospheric) pressure and are. Constant-Pressure Calorimeter Definition.
From mechasource.blogspot.com
An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas Constant-Pressure Calorimeter Definition In that case, the gaseous pressure above the solution remains constant, and. The calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Constant pressure calorimetry is used for. Constant-Pressure Calorimeter Definition.
From www.slideserve.com
PPT Zumdahl Chapter 6 PowerPoint Presentation, free download ID4269919 Constant-Pressure Calorimeter Definition Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). The calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. In that case, the gaseous pressure above the solution remains constant, and. For example, when an exothermic. A calorimeter. Constant-Pressure Calorimeter Definition.
From www.youtube.com
Constantpressure calorimetry Thermodynamics AP Chemistry Khan Constant-Pressure Calorimeter Definition The calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in. Constant-Pressure Calorimeter Definition.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID1875569 Constant-Pressure Calorimeter Definition Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). The calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. In that case, the gaseous pressure above the solution remains constant, and. For example, when an exothermic. A calorimeter. Constant-Pressure Calorimeter Definition.
From www.slideserve.com
PPT Constant Pressure Calorimetry Another Example PowerPoint Constant-Pressure Calorimeter Definition Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. In that case, the gaseous pressure above the solution remains constant, and. The. Constant-Pressure Calorimeter Definition.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Constant-Pressure Calorimeter Definition A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). In that case, the gaseous pressure above the solution remains constant, and. The calorimeters described are designed to. Constant-Pressure Calorimeter Definition.
From www.slideserve.com
PPT Constant Pressure Calorimetry Another Example PowerPoint Constant-Pressure Calorimeter Definition For example, when an exothermic. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. In that case, the gaseous pressure above the solution remains constant, and. The. Constant-Pressure Calorimeter Definition.
From saylordotorg.github.io
Calorimetry Constant-Pressure Calorimeter Definition In that case, the gaseous pressure above the solution remains constant, and. For example, when an exothermic. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). The calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. A calorimeter. Constant-Pressure Calorimeter Definition.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3850751 Constant-Pressure Calorimeter Definition For example, when an exothermic. In that case, the gaseous pressure above the solution remains constant, and. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The. Constant-Pressure Calorimeter Definition.
From www.youtube.com
AP Chemistry Thermochemistry I Part 4 Constant Pressure Calorimetry Constant-Pressure Calorimeter Definition In that case, the gaseous pressure above the solution remains constant, and. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). The calorimeters described are designed to. Constant-Pressure Calorimeter Definition.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Constant-Pressure Calorimeter Definition For example, when an exothermic. In that case, the gaseous pressure above the solution remains constant, and. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. Constant pressure calorimetry is used for. Constant-Pressure Calorimeter Definition.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID2692866 Constant-Pressure Calorimeter Definition A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. For example, when an exothermic. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is. Constant-Pressure Calorimeter Definition.
From www.slideserve.com
PPT Thermo I PowerPoint Presentation, free download ID9720008 Constant-Pressure Calorimeter Definition Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). For example, when an exothermic. The calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. A calorimeter is a device used to measure the amount of heat involved in. Constant-Pressure Calorimeter Definition.
From www.thoughtco.com
Calorimeter Definition in Chemistry Constant-Pressure Calorimeter Definition The calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. For example, when an exothermic. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). A calorimeter is a device used to measure the amount of heat involved in. Constant-Pressure Calorimeter Definition.
From www.pinterest.com
ConstantPressure Calorimetery "coffeecup" calorimeter often used to Constant-Pressure Calorimeter Definition A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. In that case, the gaseous pressure above the solution remains constant, and. Constant pressure calorimetry is used for reactions carried out under constant. Constant-Pressure Calorimeter Definition.
From app.jove.com
Constant Pressure Calorimeter/ Coffee Cup Calorimeter Concept Constant-Pressure Calorimeter Definition Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). The calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.. Constant-Pressure Calorimeter Definition.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Constant-Pressure Calorimeter Definition Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). The calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. In that case, the gaseous pressure above the solution remains constant, and. A calorimeter is a device used to. Constant-Pressure Calorimeter Definition.
From www.slideserve.com
PPT Chapter 5 Thermochemistry PowerPoint Presentation, free download Constant-Pressure Calorimeter Definition For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. In that case, the gaseous pressure above the solution remains constant, and. The calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. Constant pressure calorimetry is used for. Constant-Pressure Calorimeter Definition.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID5194704 Constant-Pressure Calorimeter Definition For example, when an exothermic. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). The calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. In that case, the gaseous pressure above the solution remains constant, and. A calorimeter. Constant-Pressure Calorimeter Definition.
From martinfersbanks.blogspot.com
Is a Bomb Calorimeter Constant Pressure Constant-Pressure Calorimeter Definition Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). The calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in. Constant-Pressure Calorimeter Definition.
From www.slideserve.com
PPT Thermodynamics 2 PowerPoint Presentation, free download ID998678 Constant-Pressure Calorimeter Definition A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). The calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow.. Constant-Pressure Calorimeter Definition.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses Constant-Pressure Calorimeter Definition Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). In that case, the gaseous pressure above the solution remains constant, and. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The. Constant-Pressure Calorimeter Definition.
From www.studocu.com
ACT 3 Constant Pressure Calorimetry ACTIVITY 3 CONSTANT PRESSURE Constant-Pressure Calorimeter Definition In that case, the gaseous pressure above the solution remains constant, and. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). The calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. For example, when an exothermic. A calorimeter. Constant-Pressure Calorimeter Definition.
From thechemistrynotes.com
Calorimeter Definition, Types and Uses Constant-Pressure Calorimeter Definition Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). The calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in. Constant-Pressure Calorimeter Definition.
From martinfersbanks.blogspot.com
Is a Bomb Calorimeter Constant Pressure Constant-Pressure Calorimeter Definition Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). For example, when an exothermic. In that case, the gaseous pressure above the solution remains constant, and. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The. Constant-Pressure Calorimeter Definition.
From martinfersbanks.blogspot.com
Is a Bomb Calorimeter Constant Pressure Constant-Pressure Calorimeter Definition A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. The calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is. Constant-Pressure Calorimeter Definition.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation Constant-Pressure Calorimeter Definition The calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q).. Constant-Pressure Calorimeter Definition.
From guweb2.gonzaga.edu
CHEM 101 General Chemistry topic Constant-Pressure Calorimeter Definition Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow.. Constant-Pressure Calorimeter Definition.
From saylordotorg.github.io
Calorimetry Constant-Pressure Calorimeter Definition A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). For example, when an exothermic. The calorimeters described are designed to operate at constant (atmospheric) pressure and are. Constant-Pressure Calorimeter Definition.
From www.researchgate.net
Schematic of constant pressure calorimeter for APW classification Constant-Pressure Calorimeter Definition Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). For example, when an exothermic. In that case, the gaseous pressure above the solution remains constant, and. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The. Constant-Pressure Calorimeter Definition.
From www.youtube.com
1A 6.7 ConstantPressure Calorimetry YouTube Constant-Pressure Calorimeter Definition In that case, the gaseous pressure above the solution remains constant, and. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). The calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. A calorimeter is a device used to. Constant-Pressure Calorimeter Definition.
From www.slideserve.com
PPT Chapter 5 PowerPoint Presentation, free download ID1549939 Constant-Pressure Calorimeter Definition A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Constant pressure calorimetry is used for reactions carried out under constant pressure when the enthalpy change (δh) is equal to the heat (q). In that case, the gaseous pressure above the solution remains constant, and. The. Constant-Pressure Calorimeter Definition.
From faqguide.co
What does a calorimeter do? Explained by FAQGuide Constant-Pressure Calorimeter Definition A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. In that case, the gaseous pressure above the solution remains constant, and. Constant pressure calorimetry is used for reactions carried out under constant. Constant-Pressure Calorimeter Definition.
From www.slideserve.com
PPT Ch 6 Thermochemistry PowerPoint Presentation, free download ID Constant-Pressure Calorimeter Definition A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. The calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. In that case, the gaseous pressure above the solution remains constant, and. Constant pressure calorimetry is used for. Constant-Pressure Calorimeter Definition.