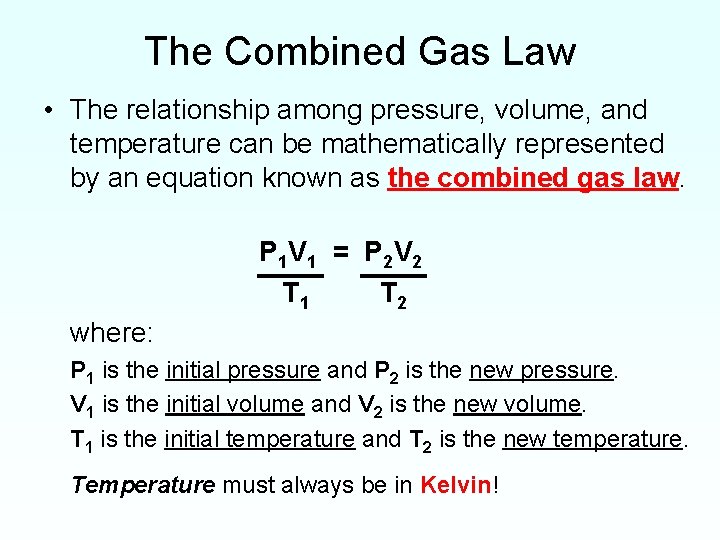
Combined Gas Law Equation For P2 . The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of. One can adjust the formula for the combined gas. So the final pressure p2, is (2.00 atm). The combined gas law states that for a fixed amount of gas: After removing the volumes, rearranging the equation: First, start with the combined gas law and cancel out the volumes because they do not change. Pvt= k (k being the proportionality constant) the combined gas law combines charles's law, boyle's law,. It is important to recognize the p total is the 98.0 value. The following equation shows how to solve. P total is the combined pressure of the dry gas and the water vapor. Therefore, the formula of combined gas law is pv/t = k, where p = pressure, t = temperature, v = volume, k is constant.
from slidetodoc.com
The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of. It is important to recognize the p total is the 98.0 value. After removing the volumes, rearranging the equation: One can adjust the formula for the combined gas. Therefore, the formula of combined gas law is pv/t = k, where p = pressure, t = temperature, v = volume, k is constant. Pvt= k (k being the proportionality constant) the combined gas law combines charles's law, boyle's law,. P total is the combined pressure of the dry gas and the water vapor. First, start with the combined gas law and cancel out the volumes because they do not change. So the final pressure p2, is (2.00 atm). The combined gas law states that for a fixed amount of gas:
The Combined Gas Law The Combined Gas Law
Combined Gas Law Equation For P2 One can adjust the formula for the combined gas. So the final pressure p2, is (2.00 atm). One can adjust the formula for the combined gas. Therefore, the formula of combined gas law is pv/t = k, where p = pressure, t = temperature, v = volume, k is constant. It is important to recognize the p total is the 98.0 value. The combined gas law states that for a fixed amount of gas: P total is the combined pressure of the dry gas and the water vapor. The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of. First, start with the combined gas law and cancel out the volumes because they do not change. After removing the volumes, rearranging the equation: Pvt= k (k being the proportionality constant) the combined gas law combines charles's law, boyle's law,. The following equation shows how to solve.
From www.slideshare.net
2 22 What Is The Combined Gas Law Combined Gas Law Equation For P2 P total is the combined pressure of the dry gas and the water vapor. First, start with the combined gas law and cancel out the volumes because they do not change. The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of. Therefore, the formula of combined gas law is pv/t =. Combined Gas Law Equation For P2.
From slidetodoc.com
The Combined Gas Law The Combined Gas Law Combined Gas Law Equation For P2 Therefore, the formula of combined gas law is pv/t = k, where p = pressure, t = temperature, v = volume, k is constant. The combined gas law states that for a fixed amount of gas: It is important to recognize the p total is the 98.0 value. The following equation shows how to solve. Pvt= k (k being the. Combined Gas Law Equation For P2.
From slideplayer.com
AGENDA 10/27/08 DO NOW (5 mins) Gas Law equation MINI LESSON(1015 Combined Gas Law Equation For P2 First, start with the combined gas law and cancel out the volumes because they do not change. The combined gas law states that for a fixed amount of gas: The following equation shows how to solve. After removing the volumes, rearranging the equation: It is important to recognize the p total is the 98.0 value. The combined gas law expresses. Combined Gas Law Equation For P2.
From owlcation.com
The Theories and Behavior of Gas Owlcation Combined Gas Law Equation For P2 Pvt= k (k being the proportionality constant) the combined gas law combines charles's law, boyle's law,. So the final pressure p2, is (2.00 atm). The combined gas law states that for a fixed amount of gas: After removing the volumes, rearranging the equation: The following equation shows how to solve. It is important to recognize the p total is the. Combined Gas Law Equation For P2.
From www.slideserve.com
PPT X Unit 14 GAS LAWS PowerPoint Presentation, free download ID Combined Gas Law Equation For P2 So the final pressure p2, is (2.00 atm). First, start with the combined gas law and cancel out the volumes because they do not change. After removing the volumes, rearranging the equation: One can adjust the formula for the combined gas. The combined gas law states that for a fixed amount of gas: The following equation shows how to solve.. Combined Gas Law Equation For P2.
From www.showme.com
ShowMe derivation of combined gas law Combined Gas Law Equation For P2 So the final pressure p2, is (2.00 atm). The following equation shows how to solve. Pvt= k (k being the proportionality constant) the combined gas law combines charles's law, boyle's law,. After removing the volumes, rearranging the equation: The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of. One can adjust. Combined Gas Law Equation For P2.
From slideplayer.com
Combined Gas Law and Avogadro’s Hypothesis ppt download Combined Gas Law Equation For P2 Pvt= k (k being the proportionality constant) the combined gas law combines charles's law, boyle's law,. The combined gas law states that for a fixed amount of gas: Therefore, the formula of combined gas law is pv/t = k, where p = pressure, t = temperature, v = volume, k is constant. P total is the combined pressure of the. Combined Gas Law Equation For P2.
From www.slideserve.com
PPT Combined Gas Law PowerPoint Presentation, free download ID3252378 Combined Gas Law Equation For P2 The following equation shows how to solve. First, start with the combined gas law and cancel out the volumes because they do not change. After removing the volumes, rearranging the equation: So the final pressure p2, is (2.00 atm). One can adjust the formula for the combined gas. The combined gas law expresses the relationship between the pressure, volume, and. Combined Gas Law Equation For P2.
From learningclignensembleu9.z22.web.core.windows.net
Combined Gas Law Explanation Combined Gas Law Equation For P2 The combined gas law states that for a fixed amount of gas: After removing the volumes, rearranging the equation: It is important to recognize the p total is the 98.0 value. The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of. First, start with the combined gas law and cancel out. Combined Gas Law Equation For P2.
From slideplayer.com
Chapter 6 Gases 6.6 The Combined Gas Law. ppt download Combined Gas Law Equation For P2 One can adjust the formula for the combined gas. It is important to recognize the p total is the 98.0 value. P total is the combined pressure of the dry gas and the water vapor. After removing the volumes, rearranging the equation: The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount. Combined Gas Law Equation For P2.
From slideplayer.com
Combined Gas Law and Avogadro’s Hypothesis ppt download Combined Gas Law Equation For P2 First, start with the combined gas law and cancel out the volumes because they do not change. Therefore, the formula of combined gas law is pv/t = k, where p = pressure, t = temperature, v = volume, k is constant. The combined gas law states that for a fixed amount of gas: The combined gas law expresses the relationship. Combined Gas Law Equation For P2.
From slidetodoc.com
The Combined Gas Law The Combined Gas Law Combined Gas Law Equation For P2 Pvt= k (k being the proportionality constant) the combined gas law combines charles's law, boyle's law,. The following equation shows how to solve. One can adjust the formula for the combined gas. It is important to recognize the p total is the 98.0 value. P total is the combined pressure of the dry gas and the water vapor. The combined. Combined Gas Law Equation For P2.
From www.expii.com
Combined Gas Law — Overview & Calculations Expii Combined Gas Law Equation For P2 So the final pressure p2, is (2.00 atm). First, start with the combined gas law and cancel out the volumes because they do not change. The following equation shows how to solve. The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of. It is important to recognize the p total is. Combined Gas Law Equation For P2.
From slideplayer.com
Chapter Combined Gas Law and Ideal Gases. ppt download Combined Gas Law Equation For P2 Therefore, the formula of combined gas law is pv/t = k, where p = pressure, t = temperature, v = volume, k is constant. The following equation shows how to solve. So the final pressure p2, is (2.00 atm). P total is the combined pressure of the dry gas and the water vapor. The combined gas law states that for. Combined Gas Law Equation For P2.
From slideplayer.com
Combined Gas Law Equation Problems ppt download Combined Gas Law Equation For P2 It is important to recognize the p total is the 98.0 value. So the final pressure p2, is (2.00 atm). Therefore, the formula of combined gas law is pv/t = k, where p = pressure, t = temperature, v = volume, k is constant. One can adjust the formula for the combined gas. The combined gas law expresses the relationship. Combined Gas Law Equation For P2.
From www.numerade.com
SOLVED Show how to solve for each of the variables in the Combined Gas Combined Gas Law Equation For P2 The combined gas law states that for a fixed amount of gas: After removing the volumes, rearranging the equation: P total is the combined pressure of the dry gas and the water vapor. The following equation shows how to solve. The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of. Therefore,. Combined Gas Law Equation For P2.
From www.slideshare.net
Gas laws Diagrams Combined Gas Law Equation For P2 First, start with the combined gas law and cancel out the volumes because they do not change. After removing the volumes, rearranging the equation: The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of. The combined gas law states that for a fixed amount of gas: One can adjust the formula. Combined Gas Law Equation For P2.
From www.slideserve.com
PPT The Combined Gas Law PowerPoint Presentation, free download ID Combined Gas Law Equation For P2 The combined gas law states that for a fixed amount of gas: Therefore, the formula of combined gas law is pv/t = k, where p = pressure, t = temperature, v = volume, k is constant. Pvt= k (k being the proportionality constant) the combined gas law combines charles's law, boyle's law,. The following equation shows how to solve. The. Combined Gas Law Equation For P2.
From www.sliderbase.com
Gas Laws Presentation Chemistry Combined Gas Law Equation For P2 After removing the volumes, rearranging the equation: The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of. Therefore, the formula of combined gas law is pv/t = k, where p = pressure, t = temperature, v = volume, k is constant. It is important to recognize the p total is the. Combined Gas Law Equation For P2.
From learningclignensembleu9.z22.web.core.windows.net
Combined Gas Law Explained Combined Gas Law Equation For P2 So the final pressure p2, is (2.00 atm). One can adjust the formula for the combined gas. Therefore, the formula of combined gas law is pv/t = k, where p = pressure, t = temperature, v = volume, k is constant. Pvt= k (k being the proportionality constant) the combined gas law combines charles's law, boyle's law,. P total is. Combined Gas Law Equation For P2.
From inspiritvr.com
Combined Gas Law Study Guide Inspirit Learning Inc Combined Gas Law Equation For P2 It is important to recognize the p total is the 98.0 value. One can adjust the formula for the combined gas. So the final pressure p2, is (2.00 atm). After removing the volumes, rearranging the equation: First, start with the combined gas law and cancel out the volumes because they do not change. Pvt= k (k being the proportionality constant). Combined Gas Law Equation For P2.
From www.slideserve.com
PPT The General Gas Equation Combined Gas Law PowerPoint Presentation Combined Gas Law Equation For P2 The following equation shows how to solve. One can adjust the formula for the combined gas. Therefore, the formula of combined gas law is pv/t = k, where p = pressure, t = temperature, v = volume, k is constant. Pvt= k (k being the proportionality constant) the combined gas law combines charles's law, boyle's law,. It is important to. Combined Gas Law Equation For P2.
From scienceinfo.com
Combined Gas Law Formula, Derivation, Examples Combined Gas Law Equation For P2 One can adjust the formula for the combined gas. The combined gas law states that for a fixed amount of gas: Pvt= k (k being the proportionality constant) the combined gas law combines charles's law, boyle's law,. After removing the volumes, rearranging the equation: P total is the combined pressure of the dry gas and the water vapor. The combined. Combined Gas Law Equation For P2.
From esaaderiksen.blogspot.com
20+ Combined Gas Law Calculator EsaadEriksen Combined Gas Law Equation For P2 So the final pressure p2, is (2.00 atm). P total is the combined pressure of the dry gas and the water vapor. Therefore, the formula of combined gas law is pv/t = k, where p = pressure, t = temperature, v = volume, k is constant. The combined gas law states that for a fixed amount of gas: The following. Combined Gas Law Equation For P2.
From www.slideserve.com
PPT The General Gas Equation Combined Gas Law PowerPoint Presentation Combined Gas Law Equation For P2 P total is the combined pressure of the dry gas and the water vapor. One can adjust the formula for the combined gas. First, start with the combined gas law and cancel out the volumes because they do not change. It is important to recognize the p total is the 98.0 value. So the final pressure p2, is (2.00 atm).. Combined Gas Law Equation For P2.
From sciencenotes.org
Combined Gas Law Definition, Formula, Examples Combined Gas Law Equation For P2 Pvt= k (k being the proportionality constant) the combined gas law combines charles's law, boyle's law,. So the final pressure p2, is (2.00 atm). First, start with the combined gas law and cancel out the volumes because they do not change. The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of.. Combined Gas Law Equation For P2.
From slideplayer.com
Aim How do gas molecules react to pressure, volume and temperature Combined Gas Law Equation For P2 The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of. One can adjust the formula for the combined gas. It is important to recognize the p total is the 98.0 value. P total is the combined pressure of the dry gas and the water vapor. The combined gas law states that. Combined Gas Law Equation For P2.
From lessonlibcallahan.z19.web.core.windows.net
Combined Gas Law Explained Combined Gas Law Equation For P2 It is important to recognize the p total is the 98.0 value. The combined gas law states that for a fixed amount of gas: The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of. P total is the combined pressure of the dry gas and the water vapor. One can adjust. Combined Gas Law Equation For P2.
From slideplayer.com
Combined Gas Law Equation Problems ppt download Combined Gas Law Equation For P2 One can adjust the formula for the combined gas. The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of. After removing the volumes, rearranging the equation: The following equation shows how to solve. Pvt= k (k being the proportionality constant) the combined gas law combines charles's law, boyle's law,. It is. Combined Gas Law Equation For P2.
From www.slideserve.com
PPT The General Gas Equation Combined Gas Law PowerPoint Presentation Combined Gas Law Equation For P2 First, start with the combined gas law and cancel out the volumes because they do not change. Pvt= k (k being the proportionality constant) the combined gas law combines charles's law, boyle's law,. Therefore, the formula of combined gas law is pv/t = k, where p = pressure, t = temperature, v = volume, k is constant. The combined gas. Combined Gas Law Equation For P2.
From www.slideserve.com
PPT Gas Law Notes Chemistry Semester II PowerPoint Presentation, free Combined Gas Law Equation For P2 So the final pressure p2, is (2.00 atm). First, start with the combined gas law and cancel out the volumes because they do not change. The combined gas law states that for a fixed amount of gas: The following equation shows how to solve. Pvt= k (k being the proportionality constant) the combined gas law combines charles's law, boyle's law,.. Combined Gas Law Equation For P2.
From www.slideserve.com
PPT The combined gas law PowerPoint Presentation, free download ID Combined Gas Law Equation For P2 The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of. Pvt= k (k being the proportionality constant) the combined gas law combines charles's law, boyle's law,. First, start with the combined gas law and cancel out the volumes because they do not change. One can adjust the formula for the combined. Combined Gas Law Equation For P2.
From slideplayer.com
Combined Gas Law Equation Problems ppt download Combined Gas Law Equation For P2 P total is the combined pressure of the dry gas and the water vapor. It is important to recognize the p total is the 98.0 value. The combined gas law states that for a fixed amount of gas: So the final pressure p2, is (2.00 atm). The combined gas law expresses the relationship between the pressure, volume, and absolute temperature. Combined Gas Law Equation For P2.
From www.youtube.com
Combined Gas Law (P1V1/T1 = P2V2/T2) Examples, Practice Problems Combined Gas Law Equation For P2 One can adjust the formula for the combined gas. Pvt= k (k being the proportionality constant) the combined gas law combines charles's law, boyle's law,. P total is the combined pressure of the dry gas and the water vapor. So the final pressure p2, is (2.00 atm). Therefore, the formula of combined gas law is pv/t = k, where p. Combined Gas Law Equation For P2.
From www.slideserve.com
PPT Combined Gas Law Avogadro’s Principle PowerPoint Presentation Combined Gas Law Equation For P2 Therefore, the formula of combined gas law is pv/t = k, where p = pressure, t = temperature, v = volume, k is constant. So the final pressure p2, is (2.00 atm). The following equation shows how to solve. The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of. After removing. Combined Gas Law Equation For P2.