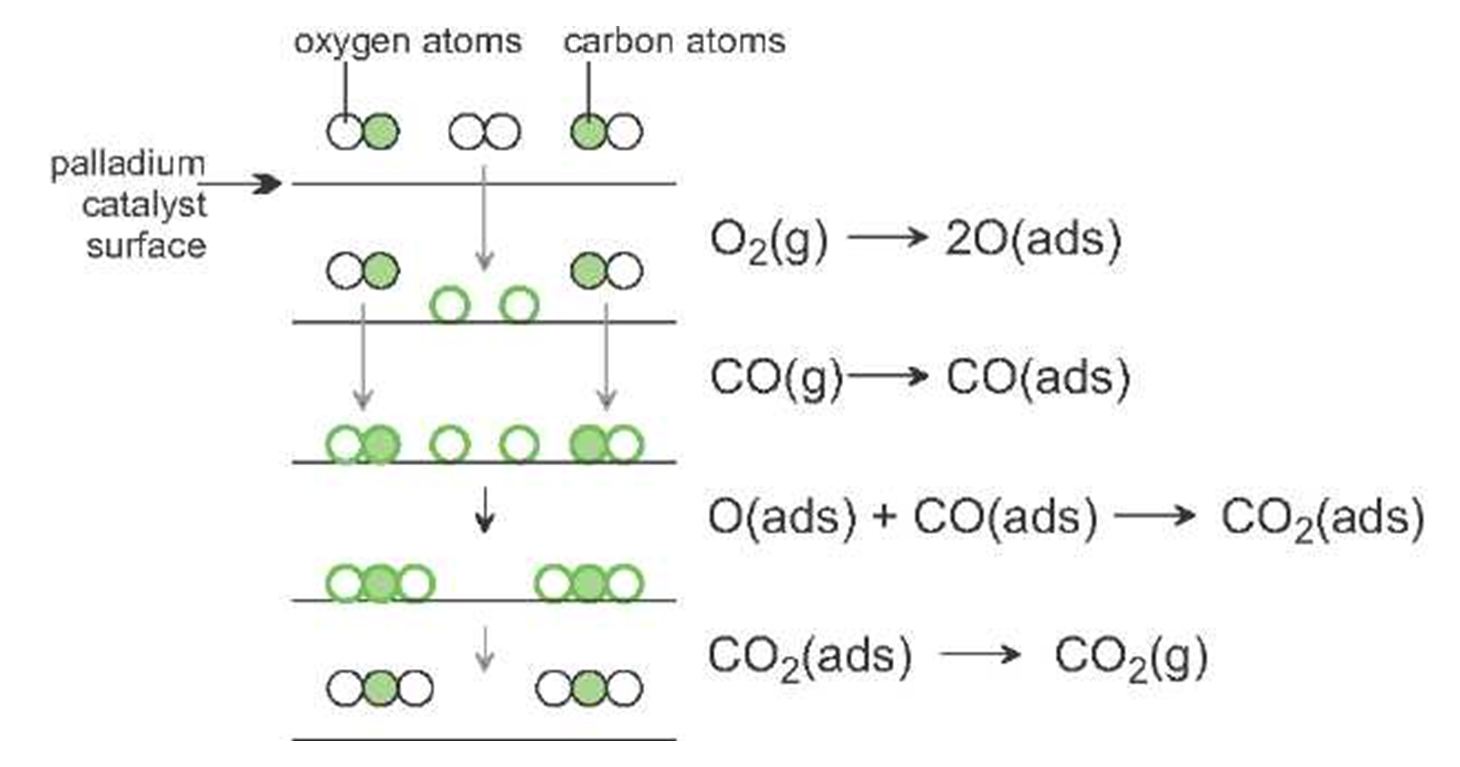
Catalysts In Chemical Reactions Examples . an example of heterogeneous catalysis is using a solid catalyst like a zeolite or alumina to catalyze a reaction in a mixture of liquids and/or. They do not appear in the reaction’s net equation and are not consumed during the. catalysts participate in a chemical reaction and increase its rate. a catalyst is a substance that is used to speed up a chemical reaction but it is not consumed by the reaction. Adding a drop of blood to a solution. The catalyst is not used up or chemically changed. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; A catalyst is a substance that speeds up a chemical reaction.
from www.essentialchemicalindustry.org
The catalyst is not used up or chemically changed. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. an example of heterogeneous catalysis is using a solid catalyst like a zeolite or alumina to catalyze a reaction in a mixture of liquids and/or. They do not appear in the reaction’s net equation and are not consumed during the. catalysts participate in a chemical reaction and increase its rate. Adding a drop of blood to a solution. A catalyst is a substance that speeds up a chemical reaction. a catalyst is a substance that is used to speed up a chemical reaction but it is not consumed by the reaction.
Catalysis in industry
Catalysts In Chemical Reactions Examples an example of heterogeneous catalysis is using a solid catalyst like a zeolite or alumina to catalyze a reaction in a mixture of liquids and/or. They do not appear in the reaction’s net equation and are not consumed during the. A catalyst is a substance that speeds up a chemical reaction. an example of heterogeneous catalysis is using a solid catalyst like a zeolite or alumina to catalyze a reaction in a mixture of liquids and/or. Adding a drop of blood to a solution. a catalyst is a substance that is used to speed up a chemical reaction but it is not consumed by the reaction. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalysts participate in a chemical reaction and increase its rate. The catalyst is not used up or chemically changed. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids;
From fyobmixiq.blob.core.windows.net
Uses Of Catalysts In Chemical Reactions at Cecilia Keffer blog Catalysts In Chemical Reactions Examples the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; They do not appear in the reaction’s net equation and are not consumed during the. A catalyst is a substance that speeds up a chemical reaction. The catalyst is not used up or chemically changed. an example of heterogeneous catalysis is using. Catalysts In Chemical Reactions Examples.
From hxeknkpwt.blob.core.windows.net
Adding A Catalyst To A Chemical Reaction Changes The Rate Of Reaction Catalysts In Chemical Reactions Examples catalysts participate in a chemical reaction and increase its rate. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; an example of heterogeneous catalysis is using a solid catalyst like a zeolite or. Catalysts In Chemical Reactions Examples.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID Catalysts In Chemical Reactions Examples They do not appear in the reaction’s net equation and are not consumed during the. The catalyst is not used up or chemically changed. Adding a drop of blood to a solution. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; catalysts participate in a chemical reaction and increase its rate.. Catalysts In Chemical Reactions Examples.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free Catalysts In Chemical Reactions Examples an example of heterogeneous catalysis is using a solid catalyst like a zeolite or alumina to catalyze a reaction in a mixture of liquids and/or. catalysts participate in a chemical reaction and increase its rate. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a substance that. Catalysts In Chemical Reactions Examples.
From www.youtube.com
Identifying catalysts in a reaction YouTube Catalysts In Chemical Reactions Examples A catalyst is a substance that speeds up a chemical reaction. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; an example of heterogeneous catalysis is using a solid catalyst like a zeolite or alumina to catalyze a reaction in a mixture of liquids and/or. They do not appear in the. Catalysts In Chemical Reactions Examples.
From hxeejbykq.blob.core.windows.net
Catalytic Reduction Equation at Lois Aguilar blog Catalysts In Chemical Reactions Examples the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; an example of heterogeneous catalysis is using a solid catalyst like a zeolite or alumina to catalyze a reaction in a mixture of liquids and/or. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed.. Catalysts In Chemical Reactions Examples.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalysts In Chemical Reactions Examples catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. an example of heterogeneous catalysis is using a solid catalyst like a zeolite or alumina to catalyze a reaction in a mixture of liquids and/or. The catalyst is not used up or chemically changed. a catalyst is a substance that is. Catalysts In Chemical Reactions Examples.
From giokgajkj.blob.core.windows.net
Catalyst Biology Def at Cleveland Bell blog Catalysts In Chemical Reactions Examples catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; catalysts participate in a chemical reaction and increase its rate. an example of heterogeneous catalysis is using a solid catalyst like a zeolite or. Catalysts In Chemical Reactions Examples.
From www.ck12.org
Catalysts Example 1 ( Video ) Chemistry CK12 Foundation Catalysts In Chemical Reactions Examples a catalyst is a substance that is used to speed up a chemical reaction but it is not consumed by the reaction. catalysts participate in a chemical reaction and increase its rate. They do not appear in the reaction’s net equation and are not consumed during the. A catalyst is a substance that speeds up a chemical reaction.. Catalysts In Chemical Reactions Examples.
From fyovoqatb.blob.core.windows.net
Catalyst Definition Example at Lorinda Starnes blog Catalysts In Chemical Reactions Examples They do not appear in the reaction’s net equation and are not consumed during the. catalysts participate in a chemical reaction and increase its rate. a catalyst is a substance that is used to speed up a chemical reaction but it is not consumed by the reaction. catalyst, in chemistry, any substance that increases the rate of. Catalysts In Chemical Reactions Examples.
From www.pinterest.com
Catalyst Easy Science in 2022 Energy activities, Chemical reactions Catalysts In Chemical Reactions Examples A catalyst is a substance that speeds up a chemical reaction. The catalyst is not used up or chemically changed. an example of heterogeneous catalysis is using a solid catalyst like a zeolite or alumina to catalyze a reaction in a mixture of liquids and/or. Adding a drop of blood to a solution. catalyst, in chemistry, any substance. Catalysts In Chemical Reactions Examples.
From exyvshuta.blob.core.windows.net
Catalyst In Chemistry Example at Sharon Kane blog Catalysts In Chemical Reactions Examples The catalyst is not used up or chemically changed. an example of heterogeneous catalysis is using a solid catalyst like a zeolite or alumina to catalyze a reaction in a mixture of liquids and/or. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; catalyst, in chemistry, any substance that increases. Catalysts In Chemical Reactions Examples.
From www.youtube.com
R2.2.6 Intermediates and catalysts (HL) YouTube Catalysts In Chemical Reactions Examples catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; a catalyst is a substance that is used to speed up a chemical reaction but it is not consumed by the reaction. The catalyst is. Catalysts In Chemical Reactions Examples.
From www.slideserve.com
PPT Factors that Affect Rate of Reaction PowerPoint Presentation Catalysts In Chemical Reactions Examples They do not appear in the reaction’s net equation and are not consumed during the. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalysts participate in a chemical reaction and increase its rate. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids;. Catalysts In Chemical Reactions Examples.
From www.slideserve.com
PPT Enzymes as Biological Catalysts PowerPoint Presentation, free Catalysts In Chemical Reactions Examples They do not appear in the reaction’s net equation and are not consumed during the. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. a catalyst is a substance that is used to speed. Catalysts In Chemical Reactions Examples.
From exyskynxj.blob.core.windows.net
List Two Examples Of Catalysts at Emanuel Johnson blog Catalysts In Chemical Reactions Examples Adding a drop of blood to a solution. catalysts participate in a chemical reaction and increase its rate. The catalyst is not used up or chemically changed. an example of heterogeneous catalysis is using a solid catalyst like a zeolite or alumina to catalyze a reaction in a mixture of liquids and/or. the most effective catalyst of. Catalysts In Chemical Reactions Examples.
From www.sciencelearn.org.nz
Chemical reactions and catalysts — Science Learning Hub Catalysts In Chemical Reactions Examples catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. They do not appear in the reaction’s net equation and are not consumed during the. The catalyst is not used up or chemically changed. a catalyst is a substance that is used to speed up a chemical reaction but it is not. Catalysts In Chemical Reactions Examples.
From circuitdiagrambomb.z21.web.core.windows.net
Catalyst Energy Diagram Catalysts In Chemical Reactions Examples Adding a drop of blood to a solution. They do not appear in the reaction’s net equation and are not consumed during the. catalysts participate in a chemical reaction and increase its rate. a catalyst is a substance that is used to speed up a chemical reaction but it is not consumed by the reaction. the most. Catalysts In Chemical Reactions Examples.
From www.tessshebaylo.com
Writing Chemical Equations Khan Academy Tessshebaylo Catalysts In Chemical Reactions Examples the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; catalysts participate in a chemical reaction and increase its rate. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. The catalyst is not used up or chemically changed. a catalyst is a substance. Catalysts In Chemical Reactions Examples.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalysts In Chemical Reactions Examples the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; A catalyst is a substance that speeds up a chemical reaction. They do not appear in the reaction’s net equation and are not consumed during the. a catalyst is a substance that is used to speed up a chemical reaction but it. Catalysts In Chemical Reactions Examples.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalysts In Chemical Reactions Examples Adding a drop of blood to a solution. a catalyst is a substance that is used to speed up a chemical reaction but it is not consumed by the reaction. an example of heterogeneous catalysis is using a solid catalyst like a zeolite or alumina to catalyze a reaction in a mixture of liquids and/or. The catalyst is. Catalysts In Chemical Reactions Examples.
From hxebeytdy.blob.core.windows.net
Heterogeneous Catalyst Chemistry Examples at Barbara Ward blog Catalysts In Chemical Reactions Examples The catalyst is not used up or chemically changed. A catalyst is a substance that speeds up a chemical reaction. a catalyst is a substance that is used to speed up a chemical reaction but it is not consumed by the reaction. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids;. Catalysts In Chemical Reactions Examples.
From brainly.in
define catalyst with example ?? Brainly.in Catalysts In Chemical Reactions Examples The catalyst is not used up or chemically changed. They do not appear in the reaction’s net equation and are not consumed during the. A catalyst is a substance that speeds up a chemical reaction. Adding a drop of blood to a solution. a catalyst is a substance that is used to speed up a chemical reaction but it. Catalysts In Chemical Reactions Examples.
From giomtjynu.blob.core.windows.net
Catalyst Co Oznacza at Betty Kirkland blog Catalysts In Chemical Reactions Examples catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. The catalyst is not used up or chemically changed. catalysts participate in a chemical reaction and increase its rate. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; an example of heterogeneous catalysis. Catalysts In Chemical Reactions Examples.
From www.slideshare.net
Catalysis Catalysts In Chemical Reactions Examples Adding a drop of blood to a solution. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; A catalyst is a substance that speeds up a chemical reaction. catalysts participate in a chemical reaction. Catalysts In Chemical Reactions Examples.
From www.slideserve.com
PPT Basic Chemistry Concepts PowerPoint Presentation, free download Catalysts In Chemical Reactions Examples catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; an example of heterogeneous catalysis is using a solid catalyst like a zeolite or alumina to catalyze a reaction in a mixture of liquids and/or.. Catalysts In Chemical Reactions Examples.
From fyobmixiq.blob.core.windows.net
Uses Of Catalysts In Chemical Reactions at Cecilia Keffer blog Catalysts In Chemical Reactions Examples an example of heterogeneous catalysis is using a solid catalyst like a zeolite or alumina to catalyze a reaction in a mixture of liquids and/or. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. They do not appear in the reaction’s net equation and are not consumed during the. The catalyst. Catalysts In Chemical Reactions Examples.
From www.slideshare.net
Catalyst Catalysts In Chemical Reactions Examples catalysts participate in a chemical reaction and increase its rate. a catalyst is a substance that is used to speed up a chemical reaction but it is not consumed by the reaction. A catalyst is a substance that speeds up a chemical reaction. The catalyst is not used up or chemically changed. catalyst, in chemistry, any substance. Catalysts In Chemical Reactions Examples.
From exyvshuta.blob.core.windows.net
Catalyst In Chemistry Example at Sharon Kane blog Catalysts In Chemical Reactions Examples catalysts participate in a chemical reaction and increase its rate. A catalyst is a substance that speeds up a chemical reaction. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. an example of. Catalysts In Chemical Reactions Examples.
From www.essentialchemicalindustry.org
Catalysis in industry Catalysts In Chemical Reactions Examples Adding a drop of blood to a solution. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; The catalyst is not used up or chemically changed. catalysts participate in a chemical reaction and increase its rate. a catalyst is a substance that is used to speed up a chemical reaction. Catalysts In Chemical Reactions Examples.
From www.youtube.com
How To Identify The Intermediate & Catalyst In a Reaction Mechanism Catalysts In Chemical Reactions Examples a catalyst is a substance that is used to speed up a chemical reaction but it is not consumed by the reaction. an example of heterogeneous catalysis is using a solid catalyst like a zeolite or alumina to catalyze a reaction in a mixture of liquids and/or. Adding a drop of blood to a solution. the most. Catalysts In Chemical Reactions Examples.
From operaresidences.com.au
What does biological catalyst mean? Opera Residences Catalysts In Chemical Reactions Examples They do not appear in the reaction’s net equation and are not consumed during the. A catalyst is a substance that speeds up a chemical reaction. The catalyst is not used up or chemically changed. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; a catalyst is a substance that is. Catalysts In Chemical Reactions Examples.
From gioxmynmb.blob.core.windows.net
Examples Of Catalysts In The Human Body at Richard Holtz blog Catalysts In Chemical Reactions Examples an example of heterogeneous catalysis is using a solid catalyst like a zeolite or alumina to catalyze a reaction in a mixture of liquids and/or. A catalyst is a substance that speeds up a chemical reaction. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; Adding a drop of blood to. Catalysts In Chemical Reactions Examples.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube Catalysts In Chemical Reactions Examples The catalyst is not used up or chemically changed. catalysts participate in a chemical reaction and increase its rate. an example of heterogeneous catalysis is using a solid catalyst like a zeolite or alumina to catalyze a reaction in a mixture of liquids and/or. a catalyst is a substance that is used to speed up a chemical. Catalysts In Chemical Reactions Examples.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Catalysts In Chemical Reactions Examples a catalyst is a substance that is used to speed up a chemical reaction but it is not consumed by the reaction. an example of heterogeneous catalysis is using a solid catalyst like a zeolite or alumina to catalyze a reaction in a mixture of liquids and/or. catalyst, in chemistry, any substance that increases the rate of. Catalysts In Chemical Reactions Examples.