Chlorine Higher Electronegativity Than Iodine . 103 rows electronegativity is not a uniquely defined property and may depend on the definition. Because sr lies far to the left of. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). this article contains comparison of key thermal and atomic properties of chlorine and iodine, two comparable chemical elements from the. chlorine is more reactive than iodine due to its higher electronegativity, resulting from its smaller atomic radius and stronger. Electronegativity is used to predict. electronegativity of chlorine is 3.16. The suggested values are all. why does electronegativity increase across a period? 119 rows values for electronegativity run from 0 to 4.
from www.numerade.com
a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). 103 rows electronegativity is not a uniquely defined property and may depend on the definition. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. Because sr lies far to the left of. 119 rows values for electronegativity run from 0 to 4. this article contains comparison of key thermal and atomic properties of chlorine and iodine, two comparable chemical elements from the. Electronegativity is used to predict. chlorine is more reactive than iodine due to its higher electronegativity, resulting from its smaller atomic radius and stronger. The suggested values are all. electronegativity of chlorine is 3.16.
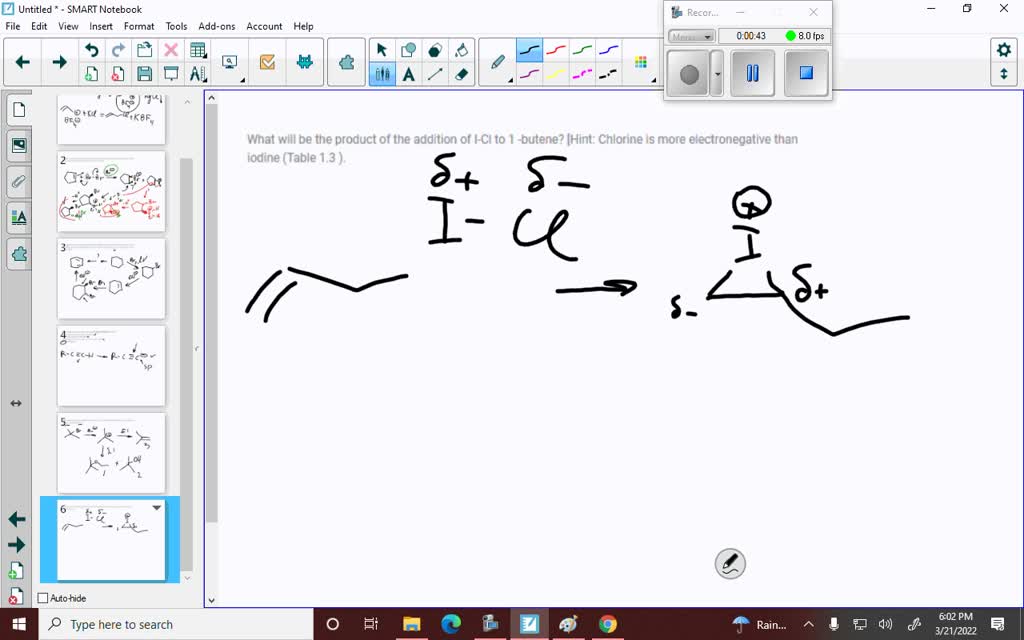
SOLVED What will be the product of the addition of ICl to 1 butene? [Hint Chlorine is more
Chlorine Higher Electronegativity Than Iodine Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. chlorine is more reactive than iodine due to its higher electronegativity, resulting from its smaller atomic radius and stronger. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. electronegativity of chlorine is 3.16. 119 rows values for electronegativity run from 0 to 4. a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). Because sr lies far to the left of. why does electronegativity increase across a period? Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. Electronegativity is used to predict. this article contains comparison of key thermal and atomic properties of chlorine and iodine, two comparable chemical elements from the. The suggested values are all.
From iperiodictable.com
What is Electronegativity Chart List of Electronegativity [PDF] Periodic Table Chlorine Higher Electronegativity Than Iodine The suggested values are all. this article contains comparison of key thermal and atomic properties of chlorine and iodine, two comparable chemical elements from the. Electronegativity is used to predict. a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). chlorine is more reactive than iodine due to its higher electronegativity, resulting. Chlorine Higher Electronegativity Than Iodine.
From www.youtube.com
ICl Lewis Structure How to Draw the Lewis Structure for the Iodine Chloride YouTube Chlorine Higher Electronegativity Than Iodine why does electronegativity increase across a period? electronegativity of chlorine is 3.16. this article contains comparison of key thermal and atomic properties of chlorine and iodine, two comparable chemical elements from the. a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). The suggested values are all. 103 rows electronegativity. Chlorine Higher Electronegativity Than Iodine.
From exocoyzqy.blob.core.windows.net
Does Chlorine Have A High Electronegativity at Gordon Maxwell blog Chlorine Higher Electronegativity Than Iodine 119 rows values for electronegativity run from 0 to 4. The suggested values are all. this article contains comparison of key thermal and atomic properties of chlorine and iodine, two comparable chemical elements from the. Because sr lies far to the left of. electronegativity of chlorine is 3.16. 103 rows electronegativity is not a uniquely defined. Chlorine Higher Electronegativity Than Iodine.
From www.researchgate.net
(a) Electronic properties of iodine, bromine, and chlorine; (b)... Download Scientific Diagram Chlorine Higher Electronegativity Than Iodine Because sr lies far to the left of. chlorine is more reactive than iodine due to its higher electronegativity, resulting from its smaller atomic radius and stronger. electronegativity of chlorine is 3.16. a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). The suggested values are all. 103 rows electronegativity is. Chlorine Higher Electronegativity Than Iodine.
From exocoyzqy.blob.core.windows.net
Does Chlorine Have A High Electronegativity at Gordon Maxwell blog Chlorine Higher Electronegativity Than Iodine Electronegativity is used to predict. chlorine is more reactive than iodine due to its higher electronegativity, resulting from its smaller atomic radius and stronger. electronegativity of chlorine is 3.16. 119 rows values for electronegativity run from 0 to 4. a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). Consider sodium. Chlorine Higher Electronegativity Than Iodine.
From www.toppr.com
Electronegativity of chlorine is three. Electron affinity of chlorine is 3.6ev/ atom. The Chlorine Higher Electronegativity Than Iodine Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). this article contains comparison of key thermal and atomic properties of chlorine and iodine, two comparable chemical elements from the. The suggested values are all. why. Chlorine Higher Electronegativity Than Iodine.
From exocoyzqy.blob.core.windows.net
Does Chlorine Have A High Electronegativity at Gordon Maxwell blog Chlorine Higher Electronegativity Than Iodine electronegativity of chlorine is 3.16. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). chlorine is more reactive than iodine due to its higher electronegativity, resulting from its smaller atomic radius and stronger. The suggested. Chlorine Higher Electronegativity Than Iodine.
From lessoncampuspolypide.z5.web.core.windows.net
Electronegativity And Polarity Chart Chlorine Higher Electronegativity Than Iodine 103 rows electronegativity is not a uniquely defined property and may depend on the definition. a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). The suggested values are all. Because sr lies far to the left of. chlorine is more reactive than iodine due to its higher electronegativity, resulting from its. Chlorine Higher Electronegativity Than Iodine.
From dxolxdofb.blob.core.windows.net
Electronegativity Chlorine And Bromine at Tammy Parker blog Chlorine Higher Electronegativity Than Iodine 103 rows electronegativity is not a uniquely defined property and may depend on the definition. Because sr lies far to the left of. why does electronegativity increase across a period? electronegativity of chlorine is 3.16. this article contains comparison of key thermal and atomic properties of chlorine and iodine, two comparable chemical elements from the. . Chlorine Higher Electronegativity Than Iodine.
From www.vrogue.co
Printable Electronegativity Periodic Table Electroneg vrogue.co Chlorine Higher Electronegativity Than Iodine why does electronegativity increase across a period? Because sr lies far to the left of. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). electronegativity of chlorine is 3.16. chlorine is more reactive than. Chlorine Higher Electronegativity Than Iodine.
From www.vedantu.com
Going from fluorine, chlorine, bromine to iodine the electronegativityA. IncreasesB. First Chlorine Higher Electronegativity Than Iodine electronegativity of chlorine is 3.16. Electronegativity is used to predict. this article contains comparison of key thermal and atomic properties of chlorine and iodine, two comparable chemical elements from the. why does electronegativity increase across a period? 103 rows electronegativity is not a uniquely defined property and may depend on the definition. 119 rows values. Chlorine Higher Electronegativity Than Iodine.
From mungfali.com
Electronegativity Trend Periodic Table Chlorine Higher Electronegativity Than Iodine Because sr lies far to the left of. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. chlorine is more reactive than iodine due to its higher electronegativity, resulting from its smaller atomic radius and stronger. 119 rows values for electronegativity run from 0 to 4. electronegativity of chlorine is. Chlorine Higher Electronegativity Than Iodine.
From mungfali.com
Atom Electronegativity Chart Chlorine Higher Electronegativity Than Iodine chlorine is more reactive than iodine due to its higher electronegativity, resulting from its smaller atomic radius and stronger. a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. this article contains comparison of key thermal. Chlorine Higher Electronegativity Than Iodine.
From dxolxdofb.blob.core.windows.net
Electronegativity Chlorine And Bromine at Tammy Parker blog Chlorine Higher Electronegativity Than Iodine 119 rows values for electronegativity run from 0 to 4. why does electronegativity increase across a period? chlorine is more reactive than iodine due to its higher electronegativity, resulting from its smaller atomic radius and stronger. The suggested values are all. Electronegativity is used to predict. 103 rows electronegativity is not a uniquely defined property and. Chlorine Higher Electronegativity Than Iodine.
From www.numerade.com
SOLVED The following chart shows the electronegativity of four halogens Halogens Chlorine Higher Electronegativity Than Iodine Electronegativity is used to predict. why does electronegativity increase across a period? 119 rows values for electronegativity run from 0 to 4. The suggested values are all. this article contains comparison of key thermal and atomic properties of chlorine and iodine, two comparable chemical elements from the. Consider sodium at the beginning of period 3 and chlorine. Chlorine Higher Electronegativity Than Iodine.
From www.numerade.com
SOLVED Sodium (Na) and hydrogen (H) have the same number of electrons in their outer shells but Chlorine Higher Electronegativity Than Iodine Because sr lies far to the left of. this article contains comparison of key thermal and atomic properties of chlorine and iodine, two comparable chemical elements from the. 119 rows values for electronegativity run from 0 to 4. electronegativity of chlorine is 3.16. The suggested values are all. 103 rows electronegativity is not a uniquely defined. Chlorine Higher Electronegativity Than Iodine.
From ecurrencythailand.com
Which Of The Following Has Highest Electron Affinity Of Fluorine Chlorine Bromine Iodine? The 5 Chlorine Higher Electronegativity Than Iodine this article contains comparison of key thermal and atomic properties of chlorine and iodine, two comparable chemical elements from the. 119 rows values for electronegativity run from 0 to 4. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. electronegativity of chlorine is 3.16. a electronegativity increases from lower. Chlorine Higher Electronegativity Than Iodine.
From www.numerade.com
SOLVED 48 S8 €8 78 C= 83 Arrange the following elements in order of increasing Chlorine Higher Electronegativity Than Iodine Because sr lies far to the left of. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). The suggested values are all. chlorine is more reactive than iodine due to its higher electronegativity, resulting from its. Chlorine Higher Electronegativity Than Iodine.
From dxoddvwxb.blob.core.windows.net
Magnesium Chloride Electronegativity at Herman blog Chlorine Higher Electronegativity Than Iodine Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. Because sr lies far to the left of. why does electronegativity increase across a period? a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). 119 rows values for electronegativity run from 0 to 4. The. Chlorine Higher Electronegativity Than Iodine.
From www.numerade.com
SOLVED What will be the product of the addition of ICl to 1 butene? [Hint Chlorine is more Chlorine Higher Electronegativity Than Iodine chlorine is more reactive than iodine due to its higher electronegativity, resulting from its smaller atomic radius and stronger. Because sr lies far to the left of. The suggested values are all. why does electronegativity increase across a period? a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). 103 rows. Chlorine Higher Electronegativity Than Iodine.
From www.nuclear-power.com
Chlorine Electron Affinity Electronegativity Ionization Energy of Chlorine Chlorine Higher Electronegativity Than Iodine why does electronegativity increase across a period? Electronegativity is used to predict. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. this article contains comparison of key thermal and atomic properties of chlorine and iodine, two comparable chemical elements from the. The suggested values are all. chlorine is more reactive. Chlorine Higher Electronegativity Than Iodine.
From www.doubtnut.com
The electronegativity of iodine is that of chlorine. (gt//lt ) Chlorine Higher Electronegativity Than Iodine The suggested values are all. this article contains comparison of key thermal and atomic properties of chlorine and iodine, two comparable chemical elements from the. Because sr lies far to the left of. why does electronegativity increase across a period? 119 rows values for electronegativity run from 0 to 4. 103 rows electronegativity is not a. Chlorine Higher Electronegativity Than Iodine.
From www.pinterest.co.uk
Illustration about Periodic table of elements with electronegativity values. Illustration of Chlorine Higher Electronegativity Than Iodine a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). 103 rows electronegativity is not a uniquely defined property and may depend on the definition. Electronegativity is used to predict. why does electronegativity increase across a period? Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the. Chlorine Higher Electronegativity Than Iodine.
From www.periodic-table.org
Chlorine Electronegativity Cl Chlorine Higher Electronegativity Than Iodine chlorine is more reactive than iodine due to its higher electronegativity, resulting from its smaller atomic radius and stronger. Electronegativity is used to predict. electronegativity of chlorine is 3.16. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. 119 rows values for electronegativity run from 0 to 4. this. Chlorine Higher Electronegativity Than Iodine.
From periodictable.me
How To Find The Electron Configuration For Chlorine Dynamic Periodic Table of Elements and Chlorine Higher Electronegativity Than Iodine this article contains comparison of key thermal and atomic properties of chlorine and iodine, two comparable chemical elements from the. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. 119 rows values for electronegativity run from 0 to 4. The suggested values are all. a electronegativity increases from lower left. Chlorine Higher Electronegativity Than Iodine.
From dxolxdofb.blob.core.windows.net
Electronegativity Chlorine And Bromine at Tammy Parker blog Chlorine Higher Electronegativity Than Iodine Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. this article contains comparison of key thermal and atomic properties of chlorine and iodine, two comparable chemical elements from the. Because sr lies far to the left of. a electronegativity increases from lower left to upper right in the periodic table (figure. Chlorine Higher Electronegativity Than Iodine.
From www.chemistrylearner.com
Electronegativity Definition, Value Chart, and Trend in Periodic Table Chlorine Higher Electronegativity Than Iodine The suggested values are all. chlorine is more reactive than iodine due to its higher electronegativity, resulting from its smaller atomic radius and stronger. why does electronegativity increase across a period? Because sr lies far to the left of. this article contains comparison of key thermal and atomic properties of chlorine and iodine, two comparable chemical elements. Chlorine Higher Electronegativity Than Iodine.
From sciencenotes.org
Halogen Elements List and Facts Chlorine Higher Electronegativity Than Iodine this article contains comparison of key thermal and atomic properties of chlorine and iodine, two comparable chemical elements from the. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. a electronegativity increases from lower left. Chlorine Higher Electronegativity Than Iodine.
From www.numerade.com
SOLVED Consider the molecules below Classify each bond as an ionic, nonpolar covalent, or Chlorine Higher Electronegativity Than Iodine electronegativity of chlorine is 3.16. 119 rows values for electronegativity run from 0 to 4. Electronegativity is used to predict. The suggested values are all. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. why does electronegativity increase across a period? this article contains comparison of key thermal and. Chlorine Higher Electronegativity Than Iodine.
From dxolxdofb.blob.core.windows.net
Electronegativity Chlorine And Bromine at Tammy Parker blog Chlorine Higher Electronegativity Than Iodine this article contains comparison of key thermal and atomic properties of chlorine and iodine, two comparable chemical elements from the. electronegativity of chlorine is 3.16. Electronegativity is used to predict. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. chlorine is more reactive than iodine due to its higher electronegativity,. Chlorine Higher Electronegativity Than Iodine.
From www.animalia-life.club
Electronegativity Periodic Table 3d Chlorine Higher Electronegativity Than Iodine electronegativity of chlorine is 3.16. 119 rows values for electronegativity run from 0 to 4. why does electronegativity increase across a period? Because sr lies far to the left of. Electronegativity is used to predict. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. a electronegativity increases from lower. Chlorine Higher Electronegativity Than Iodine.
From material-properties.org
Chlorine and Iodine Comparison Properties Material Properties Chlorine Higher Electronegativity Than Iodine 103 rows electronegativity is not a uniquely defined property and may depend on the definition. chlorine is more reactive than iodine due to its higher electronegativity, resulting from its smaller atomic radius and stronger. electronegativity of chlorine is 3.16. why does electronegativity increase across a period? 119 rows values for electronegativity run from 0 to. Chlorine Higher Electronegativity Than Iodine.
From www.dreamstime.com
Chlorine Chemical Element with 17 Atomic Number, Atomic Mass and Electronegativity Values Chlorine Higher Electronegativity Than Iodine this article contains comparison of key thermal and atomic properties of chlorine and iodine, two comparable chemical elements from the. why does electronegativity increase across a period? Electronegativity is used to predict. The suggested values are all. a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). Consider sodium at the beginning. Chlorine Higher Electronegativity Than Iodine.
From www.shutterstock.com
Electronegativity Infographic Diagram Example Sodium Chloride Stock Illustration 1084385912 Chlorine Higher Electronegativity Than Iodine this article contains comparison of key thermal and atomic properties of chlorine and iodine, two comparable chemical elements from the. The suggested values are all. why does electronegativity increase across a period? chlorine is more reactive than iodine due to its higher electronegativity, resulting from its smaller atomic radius and stronger. Because sr lies far to the. Chlorine Higher Electronegativity Than Iodine.
From www.dreamstime.com
Chlorine Chemical Element with First Ionization Energy, Atomic Mass and Electronegativity Values Chlorine Higher Electronegativity Than Iodine electronegativity of chlorine is 3.16. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. this article contains comparison of key thermal and atomic properties of chlorine and iodine, two comparable chemical elements from the. Because sr lies far to the left of. why does electronegativity increase across a period? . Chlorine Higher Electronegativity Than Iodine.