Copper Ii Oxide Colour When Heated . crystals of cuprous oxide are found in cubic shape. In the presence of more oxygen, copper(i). copper (ii) oxide is black, but when we find the percentage of oxygen in air by volume using ammonia, copper, and. When you heat the solution of cu2o in the presence of. It is insoluble in water but reacts with. for example, when copper carbonate is heated, it breaks down to produce copper oxide and carbon dioxide. the first oxidation step generates copper(i) oxide (cu 2 o), which has a reddish color. This structure is also available as a 2d mol file or as a computed 3d sd file. when hot copper oxide $\ce{cuo}$ is in contact with some ethanol vapor $\ce{c2h5oh}$, it reacts according.
from www.yaclass.in
crystals of cuprous oxide are found in cubic shape. copper (ii) oxide is black, but when we find the percentage of oxygen in air by volume using ammonia, copper, and. for example, when copper carbonate is heated, it breaks down to produce copper oxide and carbon dioxide. the first oxidation step generates copper(i) oxide (cu 2 o), which has a reddish color. When you heat the solution of cu2o in the presence of. This structure is also available as a 2d mol file or as a computed 3d sd file. when hot copper oxide $\ce{cuo}$ is in contact with some ethanol vapor $\ce{c2h5oh}$, it reacts according. It is insoluble in water but reacts with. In the presence of more oxygen, copper(i).
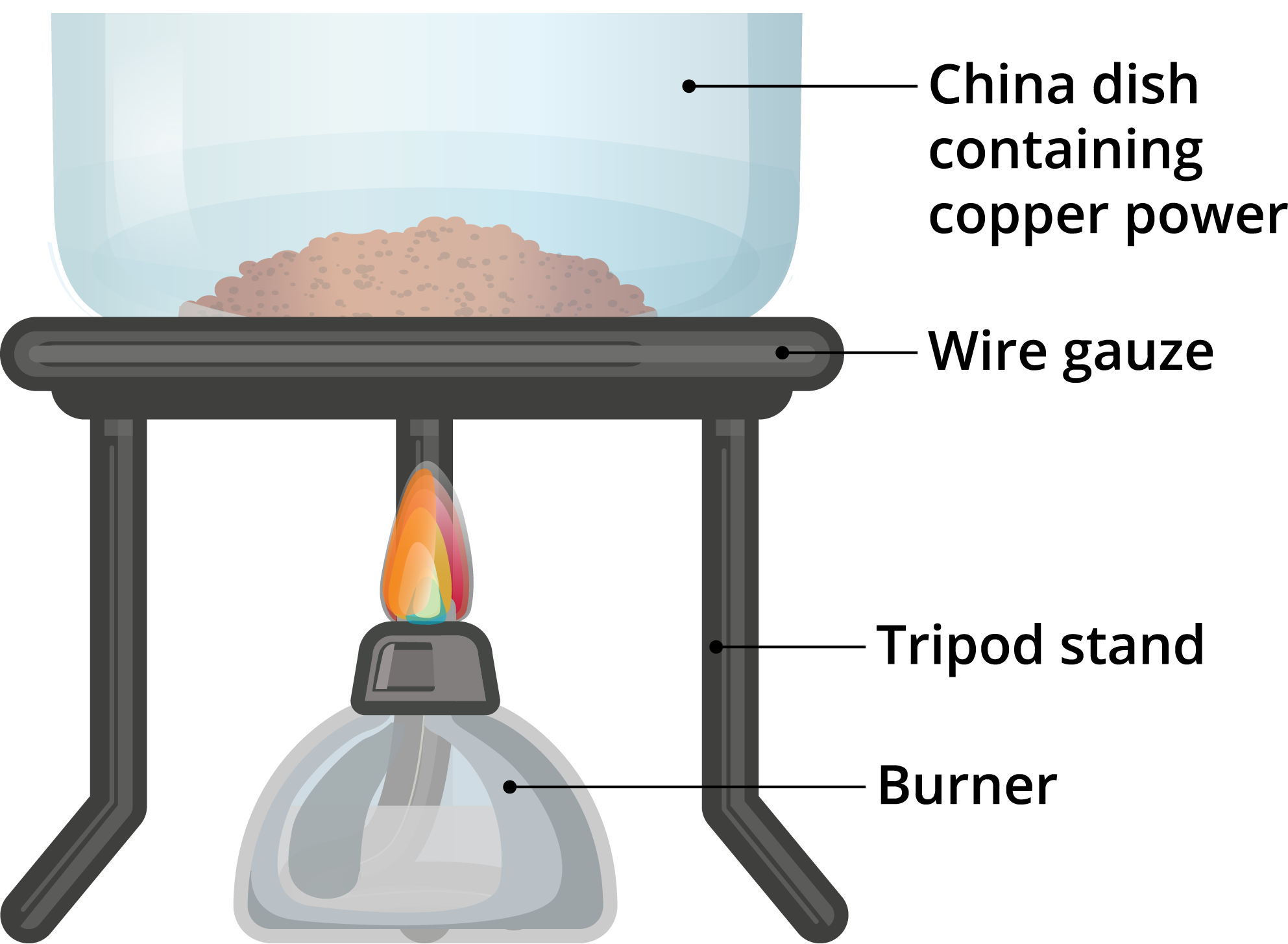
Oxidation and Reduction — lesson. Science CBSE, Class 10.
Copper Ii Oxide Colour When Heated when hot copper oxide $\ce{cuo}$ is in contact with some ethanol vapor $\ce{c2h5oh}$, it reacts according. crystals of cuprous oxide are found in cubic shape. when hot copper oxide $\ce{cuo}$ is in contact with some ethanol vapor $\ce{c2h5oh}$, it reacts according. copper (ii) oxide is black, but when we find the percentage of oxygen in air by volume using ammonia, copper, and. for example, when copper carbonate is heated, it breaks down to produce copper oxide and carbon dioxide. When you heat the solution of cu2o in the presence of. It is insoluble in water but reacts with. This structure is also available as a 2d mol file or as a computed 3d sd file. the first oxidation step generates copper(i) oxide (cu 2 o), which has a reddish color. In the presence of more oxygen, copper(i).
From www.ebay.ie
500g Black Copper (II) Oxide / Cupric Oxide CuO High Purity Grade Fine Powder eBay Copper Ii Oxide Colour When Heated This structure is also available as a 2d mol file or as a computed 3d sd file. In the presence of more oxygen, copper(i). crystals of cuprous oxide are found in cubic shape. the first oxidation step generates copper(i) oxide (cu 2 o), which has a reddish color. when hot copper oxide $\ce{cuo}$ is in contact with. Copper Ii Oxide Colour When Heated.
From www.youtube.com
Heating a mixture of carbon and copper (II) oxide YouTube Copper Ii Oxide Colour When Heated In the presence of more oxygen, copper(i). when hot copper oxide $\ce{cuo}$ is in contact with some ethanol vapor $\ce{c2h5oh}$, it reacts according. This structure is also available as a 2d mol file or as a computed 3d sd file. for example, when copper carbonate is heated, it breaks down to produce copper oxide and carbon dioxide. It. Copper Ii Oxide Colour When Heated.
From mammothmemory.net
When metals are heated it reacts with oxygen to create flame Copper Ii Oxide Colour When Heated crystals of cuprous oxide are found in cubic shape. the first oxidation step generates copper(i) oxide (cu 2 o), which has a reddish color. copper (ii) oxide is black, but when we find the percentage of oxygen in air by volume using ammonia, copper, and. This structure is also available as a 2d mol file or as. Copper Ii Oxide Colour When Heated.
From www.slideserve.com
PPT Thermal Reactions PowerPoint Presentation, free download ID2858272 Copper Ii Oxide Colour When Heated In the presence of more oxygen, copper(i). This structure is also available as a 2d mol file or as a computed 3d sd file. crystals of cuprous oxide are found in cubic shape. the first oxidation step generates copper(i) oxide (cu 2 o), which has a reddish color. copper (ii) oxide is black, but when we find. Copper Ii Oxide Colour When Heated.
From www.doubtnut.com
When hydrogen gas is passed over heated copper (II) oxide, copper and Copper Ii Oxide Colour When Heated In the presence of more oxygen, copper(i). This structure is also available as a 2d mol file or as a computed 3d sd file. It is insoluble in water but reacts with. copper (ii) oxide is black, but when we find the percentage of oxygen in air by volume using ammonia, copper, and. the first oxidation step generates. Copper Ii Oxide Colour When Heated.
From www.youtube.com
Heating a mixture of copper (II) oxide and carbon C0118 YouTube Copper Ii Oxide Colour When Heated crystals of cuprous oxide are found in cubic shape. when hot copper oxide $\ce{cuo}$ is in contact with some ethanol vapor $\ce{c2h5oh}$, it reacts according. When you heat the solution of cu2o in the presence of. This structure is also available as a 2d mol file or as a computed 3d sd file. copper (ii) oxide is. Copper Ii Oxide Colour When Heated.
From brainly.in
Colour of Copper oxide? Hehehehe Brainly.in Copper Ii Oxide Colour When Heated In the presence of more oxygen, copper(i). This structure is also available as a 2d mol file or as a computed 3d sd file. when hot copper oxide $\ce{cuo}$ is in contact with some ethanol vapor $\ce{c2h5oh}$, it reacts according. for example, when copper carbonate is heated, it breaks down to produce copper oxide and carbon dioxide. When. Copper Ii Oxide Colour When Heated.
From questions-in.kunduz.com
What happens when copper is heated in air... Physical Chemistry Copper Ii Oxide Colour When Heated When you heat the solution of cu2o in the presence of. This structure is also available as a 2d mol file or as a computed 3d sd file. for example, when copper carbonate is heated, it breaks down to produce copper oxide and carbon dioxide. when hot copper oxide $\ce{cuo}$ is in contact with some ethanol vapor $\ce{c2h5oh}$,. Copper Ii Oxide Colour When Heated.
From edu-rsc-org-s.webvpn.bjmu.doc110.com
Finding the formula of copper(II) oxide Experiment RSC Education Copper Ii Oxide Colour When Heated It is insoluble in water but reacts with. the first oxidation step generates copper(i) oxide (cu 2 o), which has a reddish color. This structure is also available as a 2d mol file or as a computed 3d sd file. crystals of cuprous oxide are found in cubic shape. when hot copper oxide $\ce{cuo}$ is in contact. Copper Ii Oxide Colour When Heated.
From blog.thepipingmart.com
Copper Oxidation States and Colors Copper Ii Oxide Colour When Heated In the presence of more oxygen, copper(i). crystals of cuprous oxide are found in cubic shape. for example, when copper carbonate is heated, it breaks down to produce copper oxide and carbon dioxide. This structure is also available as a 2d mol file or as a computed 3d sd file. the first oxidation step generates copper(i) oxide. Copper Ii Oxide Colour When Heated.
From slideplayer.com
1. ppt download Copper Ii Oxide Colour When Heated crystals of cuprous oxide are found in cubic shape. the first oxidation step generates copper(i) oxide (cu 2 o), which has a reddish color. When you heat the solution of cu2o in the presence of. In the presence of more oxygen, copper(i). when hot copper oxide $\ce{cuo}$ is in contact with some ethanol vapor $\ce{c2h5oh}$, it reacts. Copper Ii Oxide Colour When Heated.
From www.copper-powder99.com
Copper II Oxide Yosoar Copper Ii Oxide Colour When Heated when hot copper oxide $\ce{cuo}$ is in contact with some ethanol vapor $\ce{c2h5oh}$, it reacts according. the first oxidation step generates copper(i) oxide (cu 2 o), which has a reddish color. crystals of cuprous oxide are found in cubic shape. copper (ii) oxide is black, but when we find the percentage of oxygen in air by. Copper Ii Oxide Colour When Heated.
From edu.rsc.org
Reacting copper(II) oxide with sulfuric acid Experiment RSC Education Copper Ii Oxide Colour When Heated crystals of cuprous oxide are found in cubic shape. for example, when copper carbonate is heated, it breaks down to produce copper oxide and carbon dioxide. It is insoluble in water but reacts with. copper (ii) oxide is black, but when we find the percentage of oxygen in air by volume using ammonia, copper, and. When you. Copper Ii Oxide Colour When Heated.
From www.fphoto.com
Fundamental Photographs The Art of Science Copper Ii Oxide Colour When Heated when hot copper oxide $\ce{cuo}$ is in contact with some ethanol vapor $\ce{c2h5oh}$, it reacts according. When you heat the solution of cu2o in the presence of. the first oxidation step generates copper(i) oxide (cu 2 o), which has a reddish color. copper (ii) oxide is black, but when we find the percentage of oxygen in air. Copper Ii Oxide Colour When Heated.
From dxoblnjei.blob.core.windows.net
Copper Ii Oxide Is An Example Of at Bradley Bruce blog Copper Ii Oxide Colour When Heated the first oxidation step generates copper(i) oxide (cu 2 o), which has a reddish color. It is insoluble in water but reacts with. crystals of cuprous oxide are found in cubic shape. for example, when copper carbonate is heated, it breaks down to produce copper oxide and carbon dioxide. In the presence of more oxygen, copper(i). . Copper Ii Oxide Colour When Heated.
From dxoblnjei.blob.core.windows.net
Copper Ii Oxide Is An Example Of at Bradley Bruce blog Copper Ii Oxide Colour When Heated the first oxidation step generates copper(i) oxide (cu 2 o), which has a reddish color. In the presence of more oxygen, copper(i). copper (ii) oxide is black, but when we find the percentage of oxygen in air by volume using ammonia, copper, and. for example, when copper carbonate is heated, it breaks down to produce copper oxide. Copper Ii Oxide Colour When Heated.
From noahchemicals.com
4 Uses of Cupric Oxide (Copper II Oxide) Noah Chemicals Copper Ii Oxide Colour When Heated when hot copper oxide $\ce{cuo}$ is in contact with some ethanol vapor $\ce{c2h5oh}$, it reacts according. for example, when copper carbonate is heated, it breaks down to produce copper oxide and carbon dioxide. copper (ii) oxide is black, but when we find the percentage of oxygen in air by volume using ammonia, copper, and. the first. Copper Ii Oxide Colour When Heated.
From fphoto.photoshelter.com
science chemistry oxidation reaction cupric oxide Fundamental Photographs The Art of Science Copper Ii Oxide Colour When Heated It is insoluble in water but reacts with. In the presence of more oxygen, copper(i). for example, when copper carbonate is heated, it breaks down to produce copper oxide and carbon dioxide. This structure is also available as a 2d mol file or as a computed 3d sd file. When you heat the solution of cu2o in the presence. Copper Ii Oxide Colour When Heated.
From briesnitzhnschematic.z14.web.core.windows.net
Plumbing And Heating Color Codes Copper Ii Oxide Colour When Heated for example, when copper carbonate is heated, it breaks down to produce copper oxide and carbon dioxide. when hot copper oxide $\ce{cuo}$ is in contact with some ethanol vapor $\ce{c2h5oh}$, it reacts according. In the presence of more oxygen, copper(i). the first oxidation step generates copper(i) oxide (cu 2 o), which has a reddish color. It is. Copper Ii Oxide Colour When Heated.
From www.ceramic-glazes.com
CUPRIC CARBONATE Copper II Carbonate Ceramic Pigments and Stains Copper Ii Oxide Colour When Heated for example, when copper carbonate is heated, it breaks down to produce copper oxide and carbon dioxide. When you heat the solution of cu2o in the presence of. crystals of cuprous oxide are found in cubic shape. the first oxidation step generates copper(i) oxide (cu 2 o), which has a reddish color. This structure is also available. Copper Ii Oxide Colour When Heated.
From www.numerade.com
When copper(II) oxide is heated in the presence of hydrogen gas, elemental copper and water are Copper Ii Oxide Colour When Heated In the presence of more oxygen, copper(i). the first oxidation step generates copper(i) oxide (cu 2 o), which has a reddish color. When you heat the solution of cu2o in the presence of. It is insoluble in water but reacts with. This structure is also available as a 2d mol file or as a computed 3d sd file. . Copper Ii Oxide Colour When Heated.
From www.researchgate.net
TEM images of copper(I,II) oxide clusters and copper(II) oxide... Download Scientific Diagram Copper Ii Oxide Colour When Heated When you heat the solution of cu2o in the presence of. when hot copper oxide $\ce{cuo}$ is in contact with some ethanol vapor $\ce{c2h5oh}$, it reacts according. In the presence of more oxygen, copper(i). It is insoluble in water but reacts with. This structure is also available as a 2d mol file or as a computed 3d sd file.. Copper Ii Oxide Colour When Heated.
From www.slideshare.net
Action of heat on chemical compound e book Copper Ii Oxide Colour When Heated When you heat the solution of cu2o in the presence of. This structure is also available as a 2d mol file or as a computed 3d sd file. the first oxidation step generates copper(i) oxide (cu 2 o), which has a reddish color. In the presence of more oxygen, copper(i). crystals of cuprous oxide are found in cubic. Copper Ii Oxide Colour When Heated.
From dxoblnjei.blob.core.windows.net
Copper Ii Oxide Is An Example Of at Bradley Bruce blog Copper Ii Oxide Colour When Heated In the presence of more oxygen, copper(i). when hot copper oxide $\ce{cuo}$ is in contact with some ethanol vapor $\ce{c2h5oh}$, it reacts according. It is insoluble in water but reacts with. When you heat the solution of cu2o in the presence of. This structure is also available as a 2d mol file or as a computed 3d sd file.. Copper Ii Oxide Colour When Heated.
From mavink.com
Different Types Of Copper Copper Ii Oxide Colour When Heated This structure is also available as a 2d mol file or as a computed 3d sd file. crystals of cuprous oxide are found in cubic shape. when hot copper oxide $\ce{cuo}$ is in contact with some ethanol vapor $\ce{c2h5oh}$, it reacts according. In the presence of more oxygen, copper(i). for example, when copper carbonate is heated, it. Copper Ii Oxide Colour When Heated.
From exotsdtks.blob.core.windows.net
Copper (Ii) Oxide Formula at Travis Summers blog Copper Ii Oxide Colour When Heated for example, when copper carbonate is heated, it breaks down to produce copper oxide and carbon dioxide. copper (ii) oxide is black, but when we find the percentage of oxygen in air by volume using ammonia, copper, and. the first oxidation step generates copper(i) oxide (cu 2 o), which has a reddish color. when hot copper. Copper Ii Oxide Colour When Heated.
From www.coursehero.com
[Solved] 1. When solid copper (II) oxide is heated in the presence of... Course Hero Copper Ii Oxide Colour When Heated copper (ii) oxide is black, but when we find the percentage of oxygen in air by volume using ammonia, copper, and. crystals of cuprous oxide are found in cubic shape. In the presence of more oxygen, copper(i). It is insoluble in water but reacts with. when hot copper oxide $\ce{cuo}$ is in contact with some ethanol vapor. Copper Ii Oxide Colour When Heated.
From www.youtube.com
Chemical Properties of Hydrogen Reaction of hydrogen with copper II oxide YouTube Copper Ii Oxide Colour When Heated copper (ii) oxide is black, but when we find the percentage of oxygen in air by volume using ammonia, copper, and. crystals of cuprous oxide are found in cubic shape. the first oxidation step generates copper(i) oxide (cu 2 o), which has a reddish color. This structure is also available as a 2d mol file or as. Copper Ii Oxide Colour When Heated.
From fphoto.photoshelter.com
science chemical reaction equilibrium cupric carbonate Fundamental Photographs The Art of Copper Ii Oxide Colour When Heated the first oxidation step generates copper(i) oxide (cu 2 o), which has a reddish color. When you heat the solution of cu2o in the presence of. It is insoluble in water but reacts with. when hot copper oxide $\ce{cuo}$ is in contact with some ethanol vapor $\ce{c2h5oh}$, it reacts according. for example, when copper carbonate is heated,. Copper Ii Oxide Colour When Heated.
From exospybey.blob.core.windows.net
Copper Ii Oxide And Carbon Dioxide at Carmen Gadson blog Copper Ii Oxide Colour When Heated In the presence of more oxygen, copper(i). When you heat the solution of cu2o in the presence of. This structure is also available as a 2d mol file or as a computed 3d sd file. copper (ii) oxide is black, but when we find the percentage of oxygen in air by volume using ammonia, copper, and. for example,. Copper Ii Oxide Colour When Heated.
From blog.adafruit.com
The Color Spectrum of Heated Steel « Adafruit Industries Makers, hackers, artists, designers Copper Ii Oxide Colour When Heated It is insoluble in water but reacts with. When you heat the solution of cu2o in the presence of. the first oxidation step generates copper(i) oxide (cu 2 o), which has a reddish color. when hot copper oxide $\ce{cuo}$ is in contact with some ethanol vapor $\ce{c2h5oh}$, it reacts according. for example, when copper carbonate is heated,. Copper Ii Oxide Colour When Heated.
From www.toppr.com
On heating blue coloured powder of copper (II) nitrate in a boiling tube, black copper oxide, O2 Copper Ii Oxide Colour When Heated for example, when copper carbonate is heated, it breaks down to produce copper oxide and carbon dioxide. when hot copper oxide $\ce{cuo}$ is in contact with some ethanol vapor $\ce{c2h5oh}$, it reacts according. copper (ii) oxide is black, but when we find the percentage of oxygen in air by volume using ammonia, copper, and. In the presence. Copper Ii Oxide Colour When Heated.
From www.yaclass.in
Oxidation and Reduction — lesson. Science CBSE, Class 10. Copper Ii Oxide Colour When Heated When you heat the solution of cu2o in the presence of. In the presence of more oxygen, copper(i). crystals of cuprous oxide are found in cubic shape. This structure is also available as a 2d mol file or as a computed 3d sd file. It is insoluble in water but reacts with. copper (ii) oxide is black, but. Copper Ii Oxide Colour When Heated.
From www.numerade.com
SOLVED When copper (II) oxide is heated in the presence of hydrogen gas, elemental copper and Copper Ii Oxide Colour When Heated In the presence of more oxygen, copper(i). when hot copper oxide $\ce{cuo}$ is in contact with some ethanol vapor $\ce{c2h5oh}$, it reacts according. When you heat the solution of cu2o in the presence of. for example, when copper carbonate is heated, it breaks down to produce copper oxide and carbon dioxide. This structure is also available as a. Copper Ii Oxide Colour When Heated.
From www.toppr.com
On heating blue coloured powder of copper (II) nitrate in a boiling tube, copper oxide, a Copper Ii Oxide Colour When Heated when hot copper oxide $\ce{cuo}$ is in contact with some ethanol vapor $\ce{c2h5oh}$, it reacts according. When you heat the solution of cu2o in the presence of. It is insoluble in water but reacts with. for example, when copper carbonate is heated, it breaks down to produce copper oxide and carbon dioxide. the first oxidation step generates. Copper Ii Oxide Colour When Heated.