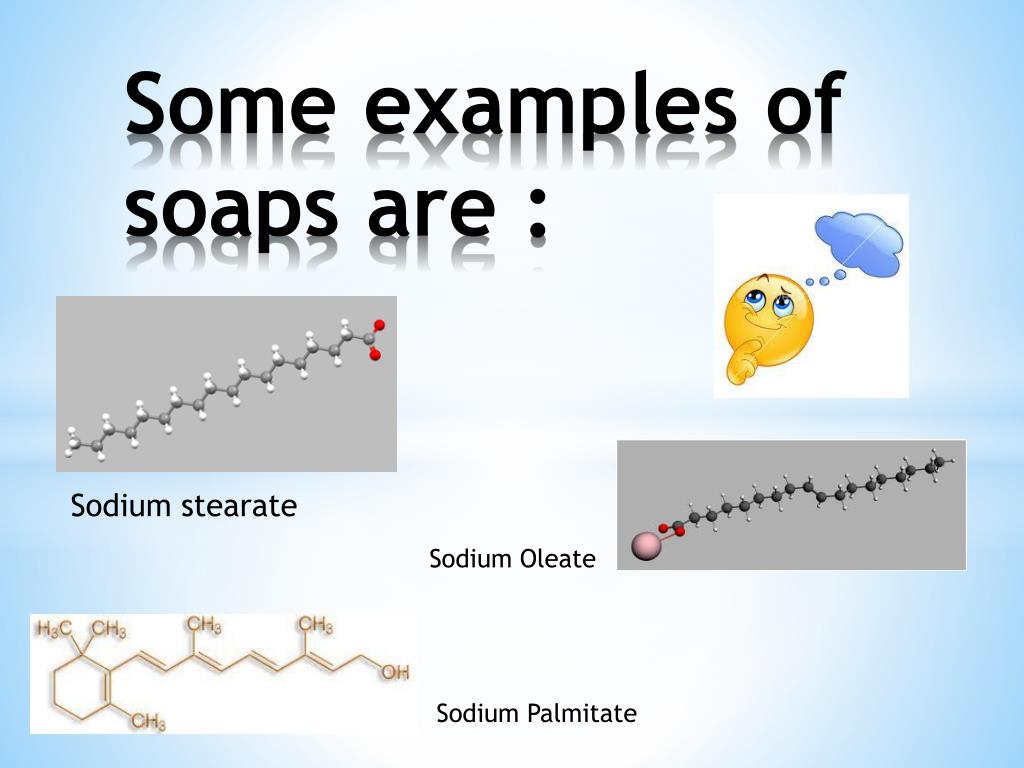
Soaps Are Sodium Salts Of . soap is a salt of a fatty acid [1] used in a variety of cleansing and lubricating products. The presence of a strong base. Most of the dirt is oily in nature and oil does not dissolve in water. soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. saponification (production of soaps) is the hydrolysis of triglycerides (fats) using a strong base such as naoh instead of water. Soaps are cleaning agents or detergents. Molecules of soap are made up of two. In a domestic setting, soaps are surfactants usually used. soft potassium soaps were then converted to the harder sodium soaps by washing with salt solution. soaps are potassium or sodium salts of a carboxylic acid having a long aliphatic chain attached to it. The importance of soap to. cleansing action of soaps and detergents. They are surfactants (compounds that reduce.
from www.slideserve.com
The presence of a strong base. Molecules of soap are made up of two. Soaps are cleaning agents or detergents. soap is a salt of a fatty acid [1] used in a variety of cleansing and lubricating products. Most of the dirt is oily in nature and oil does not dissolve in water. The importance of soap to. soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. saponification (production of soaps) is the hydrolysis of triglycerides (fats) using a strong base such as naoh instead of water. In a domestic setting, soaps are surfactants usually used. soft potassium soaps were then converted to the harder sodium soaps by washing with salt solution.
PPT SOAPS AND DETERGENTS PowerPoint Presentation, free download ID1978873
Soaps Are Sodium Salts Of soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. soap is a salt of a fatty acid [1] used in a variety of cleansing and lubricating products. Soaps are cleaning agents or detergents. soaps are potassium or sodium salts of a carboxylic acid having a long aliphatic chain attached to it. saponification (production of soaps) is the hydrolysis of triglycerides (fats) using a strong base such as naoh instead of water. The importance of soap to. soft potassium soaps were then converted to the harder sodium soaps by washing with salt solution. soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Most of the dirt is oily in nature and oil does not dissolve in water. They are surfactants (compounds that reduce. In a domestic setting, soaps are surfactants usually used. cleansing action of soaps and detergents. The presence of a strong base. Molecules of soap are made up of two.
From standardsalts.com
Himalayan Salt Soap Properties, Benefits, Uses Standard Salts Soaps Are Sodium Salts Of soaps are potassium or sodium salts of a carboxylic acid having a long aliphatic chain attached to it. Soaps are cleaning agents or detergents. soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. soap is a salt of a fatty acid [1] used in a variety. Soaps Are Sodium Salts Of.
From www.teachoo.com
[Class 10] Soaps and Detergents Structure, Cleansing Action and more Soaps Are Sodium Salts Of Soaps are cleaning agents or detergents. The importance of soap to. saponification (production of soaps) is the hydrolysis of triglycerides (fats) using a strong base such as naoh instead of water. Molecules of soap are made up of two. soft potassium soaps were then converted to the harder sodium soaps by washing with salt solution. cleansing action. Soaps Are Sodium Salts Of.
From www.slideserve.com
PPT Soap and Detergents Manufacture PowerPoint Presentation ID2067968 Soaps Are Sodium Salts Of soaps are potassium or sodium salts of a carboxylic acid having a long aliphatic chain attached to it. Most of the dirt is oily in nature and oil does not dissolve in water. cleansing action of soaps and detergents. The importance of soap to. The presence of a strong base. soft potassium soaps were then converted to. Soaps Are Sodium Salts Of.
From niftywellness.com
The Amazing Benefits Of Salt Soap & Easy DIY Guide Soaps Are Sodium Salts Of Most of the dirt is oily in nature and oil does not dissolve in water. The presence of a strong base. soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Soaps are cleaning agents or detergents. The importance of soap to. In a domestic setting, soaps are surfactants. Soaps Are Sodium Salts Of.
From www.pinterest.com
How to Use Sodium Lactate Soaps Are Sodium Salts Of They are surfactants (compounds that reduce. soft potassium soaps were then converted to the harder sodium soaps by washing with salt solution. soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. saponification (production of soaps) is the hydrolysis of triglycerides (fats) using a strong base such. Soaps Are Sodium Salts Of.
From niftywellness.com
The Amazing Benefits Of Salt Soap & Easy DIY Guide Soaps Are Sodium Salts Of In a domestic setting, soaps are surfactants usually used. saponification (production of soaps) is the hydrolysis of triglycerides (fats) using a strong base such as naoh instead of water. The presence of a strong base. The importance of soap to. Soaps are cleaning agents or detergents. Most of the dirt is oily in nature and oil does not dissolve. Soaps Are Sodium Salts Of.
From stock.adobe.com
Structural chemical formula of sodium laurate molecule with soaps. Sodium laurate (or Soaps Are Sodium Salts Of soft potassium soaps were then converted to the harder sodium soaps by washing with salt solution. The importance of soap to. Most of the dirt is oily in nature and oil does not dissolve in water. saponification (production of soaps) is the hydrolysis of triglycerides (fats) using a strong base such as naoh instead of water. soaps. Soaps Are Sodium Salts Of.
From www.alamy.com
General formula of solid soap molecule. Sodium carboxylate, RCOONa. It is the sodium salt of Soaps Are Sodium Salts Of soaps are potassium or sodium salts of a carboxylic acid having a long aliphatic chain attached to it. soap is a salt of a fatty acid [1] used in a variety of cleansing and lubricating products. The importance of soap to. Molecules of soap are made up of two. They are surfactants (compounds that reduce. saponification (production. Soaps Are Sodium Salts Of.
From www.soapqueen.com
How to Use and Thicken Liquid Soap Base Soap Queen Soaps Are Sodium Salts Of saponification (production of soaps) is the hydrolysis of triglycerides (fats) using a strong base such as naoh instead of water. The importance of soap to. In a domestic setting, soaps are surfactants usually used. soaps are potassium or sodium salts of a carboxylic acid having a long aliphatic chain attached to it. cleansing action of soaps and. Soaps Are Sodium Salts Of.
From www.pinterest.com
Salt, Sugar, and Sodium Lactate in Soap Countryside Lactation, Natural body care, Soap Soaps Are Sodium Salts Of Soaps are cleaning agents or detergents. soap is a salt of a fatty acid [1] used in a variety of cleansing and lubricating products. cleansing action of soaps and detergents. The importance of soap to. They are surfactants (compounds that reduce. Most of the dirt is oily in nature and oil does not dissolve in water. The presence. Soaps Are Sodium Salts Of.
From www.slideserve.com
PPT Preparation and Properties of a Soap PowerPoint Presentation, free download ID1705250 Soaps Are Sodium Salts Of Most of the dirt is oily in nature and oil does not dissolve in water. Molecules of soap are made up of two. soaps are potassium or sodium salts of a carboxylic acid having a long aliphatic chain attached to it. Soaps are cleaning agents or detergents. soft potassium soaps were then converted to the harder sodium soaps. Soaps Are Sodium Salts Of.
From stock.adobe.com
Structural chemical formula of sodium laurate molecule with soaps. Sodium laurate (or Soaps Are Sodium Salts Of The presence of a strong base. Most of the dirt is oily in nature and oil does not dissolve in water. Soaps are cleaning agents or detergents. In a domestic setting, soaps are surfactants usually used. The importance of soap to. Molecules of soap are made up of two. soft potassium soaps were then converted to the harder sodium. Soaps Are Sodium Salts Of.
From www.thoughtco.com
How Saponification Makes Soap Soaps Are Sodium Salts Of The presence of a strong base. saponification (production of soaps) is the hydrolysis of triglycerides (fats) using a strong base such as naoh instead of water. soft potassium soaps were then converted to the harder sodium soaps by washing with salt solution. soap is a salt of a fatty acid [1] used in a variety of cleansing. Soaps Are Sodium Salts Of.
From bossanovasoap.com
CHEMISTRY Soaps Are Sodium Salts Of soap is a salt of a fatty acid [1] used in a variety of cleansing and lubricating products. The presence of a strong base. The importance of soap to. cleansing action of soaps and detergents. Soaps are cleaning agents or detergents. soft potassium soaps were then converted to the harder sodium soaps by washing with salt solution.. Soaps Are Sodium Salts Of.
From spmchemistry.blog.onlinetuition.com.my
The Making of Detergent SPM Chemistry Soaps Are Sodium Salts Of Soaps are cleaning agents or detergents. The importance of soap to. Molecules of soap are made up of two. soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Most of the dirt is oily in nature and oil does not dissolve in water. They are surfactants (compounds that. Soaps Are Sodium Salts Of.
From slideplayer.com
CHAPTER 4 CARBON AND ITS COMPOUNDS ppt download Soaps Are Sodium Salts Of The importance of soap to. saponification (production of soaps) is the hydrolysis of triglycerides (fats) using a strong base such as naoh instead of water. cleansing action of soaps and detergents. They are surfactants (compounds that reduce. Soaps are cleaning agents or detergents. soaps are potassium or sodium salts of a carboxylic acid having a long aliphatic. Soaps Are Sodium Salts Of.
From www.slideserve.com
PPT SOAPS AND DETERGENTS PowerPoint Presentation, free download ID1978873 Soaps Are Sodium Salts Of soft potassium soaps were then converted to the harder sodium soaps by washing with salt solution. cleansing action of soaps and detergents. Most of the dirt is oily in nature and oil does not dissolve in water. The presence of a strong base. soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats. Soaps Are Sodium Salts Of.
From ar.inspiredpencil.com
Sodium Hydroxide Soap Soaps Are Sodium Salts Of The importance of soap to. Soaps are cleaning agents or detergents. The presence of a strong base. soaps are potassium or sodium salts of a carboxylic acid having a long aliphatic chain attached to it. Molecules of soap are made up of two. soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in. Soaps Are Sodium Salts Of.
From soapdelinews.com
Pink Salt and Sal Butter Soap Recipe Soap Deli News Soaps Are Sodium Salts Of soaps are potassium or sodium salts of a carboxylic acid having a long aliphatic chain attached to it. Molecules of soap are made up of two. In a domestic setting, soaps are surfactants usually used. cleansing action of soaps and detergents. saponification (production of soaps) is the hydrolysis of triglycerides (fats) using a strong base such as. Soaps Are Sodium Salts Of.
From www.alamy.com
Soaps are sodium or potassium salts of fatty acids which are formed by hydrolysis of fats and Soaps Are Sodium Salts Of Soaps are cleaning agents or detergents. soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. The importance of soap to. Molecules of soap are made up of two. saponification (production of soaps) is the hydrolysis of triglycerides (fats) using a strong base such as naoh instead of. Soaps Are Sodium Salts Of.
From askfilo.com
commercially used soaps are sodium salts of fatty a tearic acid (C17 H35 Soaps Are Sodium Salts Of The presence of a strong base. soap is a salt of a fatty acid [1] used in a variety of cleansing and lubricating products. soft potassium soaps were then converted to the harder sodium soaps by washing with salt solution. Molecules of soap are made up of two. Soaps are cleaning agents or detergents. soaps are sodium. Soaps Are Sodium Salts Of.
From valproco.com
ValPro 59 Sodium Cocoate & Sodium Vegate Soap Powder Valley Products Co. Soaps Are Sodium Salts Of In a domestic setting, soaps are surfactants usually used. Soaps are cleaning agents or detergents. soft potassium soaps were then converted to the harder sodium soaps by washing with salt solution. cleansing action of soaps and detergents. Molecules of soap are made up of two. soap is a salt of a fatty acid [1] used in a. Soaps Are Sodium Salts Of.
From www.shutterstock.com
Soap Soaps Sodium Potassium Salts Long Stock Vector (Royalty Free) 2157875643 Shutterstock Soaps Are Sodium Salts Of In a domestic setting, soaps are surfactants usually used. The presence of a strong base. They are surfactants (compounds that reduce. soaps are potassium or sodium salts of a carboxylic acid having a long aliphatic chain attached to it. soap is a salt of a fatty acid [1] used in a variety of cleansing and lubricating products. Most. Soaps Are Sodium Salts Of.
From www.pinterest.com
Buy and Save on Sodium Lauryl Sulfoacetate Slsa 1 Lb powder for all of your favorite bath time Soaps Are Sodium Salts Of saponification (production of soaps) is the hydrolysis of triglycerides (fats) using a strong base such as naoh instead of water. In a domestic setting, soaps are surfactants usually used. Most of the dirt is oily in nature and oil does not dissolve in water. soft potassium soaps were then converted to the harder sodium soaps by washing with. Soaps Are Sodium Salts Of.
From www.scribd.com
Soap Soap Sodium Hydroxide Soaps Are Sodium Salts Of soaps are potassium or sodium salts of a carboxylic acid having a long aliphatic chain attached to it. soft potassium soaps were then converted to the harder sodium soaps by washing with salt solution. soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Molecules of soap. Soaps Are Sodium Salts Of.
From www.slideserve.com
PPT SOAPS AND DETERGENTS PowerPoint Presentation, free download ID1978873 Soaps Are Sodium Salts Of Soaps are cleaning agents or detergents. soap is a salt of a fatty acid [1] used in a variety of cleansing and lubricating products. In a domestic setting, soaps are surfactants usually used. The importance of soap to. soaps are potassium or sodium salts of a carboxylic acid having a long aliphatic chain attached to it. They are. Soaps Are Sodium Salts Of.
From raksam.com
Soap Noodles Raksam Ingredients Soaps Are Sodium Salts Of soap is a salt of a fatty acid [1] used in a variety of cleansing and lubricating products. Molecules of soap are made up of two. soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. saponification (production of soaps) is the hydrolysis of triglycerides (fats) using. Soaps Are Sodium Salts Of.
From www.dreamstime.com
General Formula of Solid Soap Molecule. Sodium Carboxylate, RCOONa. it is the Sodium Salt of Soaps Are Sodium Salts Of Molecules of soap are made up of two. soap is a salt of a fatty acid [1] used in a variety of cleansing and lubricating products. They are surfactants (compounds that reduce. soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. The presence of a strong base.. Soaps Are Sodium Salts Of.
From ar.inspiredpencil.com
Soap Molecule Structure Soaps Are Sodium Salts Of soaps are potassium or sodium salts of a carboxylic acid having a long aliphatic chain attached to it. cleansing action of soaps and detergents. saponification (production of soaps) is the hydrolysis of triglycerides (fats) using a strong base such as naoh instead of water. Molecules of soap are made up of two. The presence of a strong. Soaps Are Sodium Salts Of.
From slideplayer.com
SOAPS AND DETERGENTS. ppt download Soaps Are Sodium Salts Of cleansing action of soaps and detergents. soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. saponification (production of soaps) is the hydrolysis of triglycerides (fats) using a strong base such as naoh instead of water. soap is a salt of a fatty acid [1] used. Soaps Are Sodium Salts Of.
From makingsoapnaturally.com
Salt Soap Recipe Making Soap NaturallyMaking Soap Naturally Soaps Are Sodium Salts Of Soaps are cleaning agents or detergents. Most of the dirt is oily in nature and oil does not dissolve in water. cleansing action of soaps and detergents. Molecules of soap are made up of two. In a domestic setting, soaps are surfactants usually used. saponification (production of soaps) is the hydrolysis of triglycerides (fats) using a strong base. Soaps Are Sodium Salts Of.
From niftywellness.com
The Amazing Benefits Of Salt Soap & Easy DIY Guide Soaps Are Sodium Salts Of Molecules of soap are made up of two. cleansing action of soaps and detergents. In a domestic setting, soaps are surfactants usually used. The importance of soap to. The presence of a strong base. soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. They are surfactants (compounds. Soaps Are Sodium Salts Of.
From www.slideserve.com
PPT SOAPS AND DETERGENTS PowerPoint Presentation, free download ID1978873 Soaps Are Sodium Salts Of The importance of soap to. The presence of a strong base. saponification (production of soaps) is the hydrolysis of triglycerides (fats) using a strong base such as naoh instead of water. soaps are potassium or sodium salts of a carboxylic acid having a long aliphatic chain attached to it. Most of the dirt is oily in nature and. Soaps Are Sodium Salts Of.
From www.shutterstock.com
Structural Chemical Formula Sodium Laurate Molecule Stock Photo 1836763807 Shutterstock Soaps Are Sodium Salts Of saponification (production of soaps) is the hydrolysis of triglycerides (fats) using a strong base such as naoh instead of water. Most of the dirt is oily in nature and oil does not dissolve in water. Soaps are cleaning agents or detergents. Molecules of soap are made up of two. The importance of soap to. soft potassium soaps were. Soaps Are Sodium Salts Of.
From diynatural.com
Soap Salts A DIY Salt Soap Recipe and A DIY Brine Soap Recipe Soaps Are Sodium Salts Of Soaps are cleaning agents or detergents. The presence of a strong base. They are surfactants (compounds that reduce. soaps are potassium or sodium salts of a carboxylic acid having a long aliphatic chain attached to it. soft potassium soaps were then converted to the harder sodium soaps by washing with salt solution. cleansing action of soaps and. Soaps Are Sodium Salts Of.