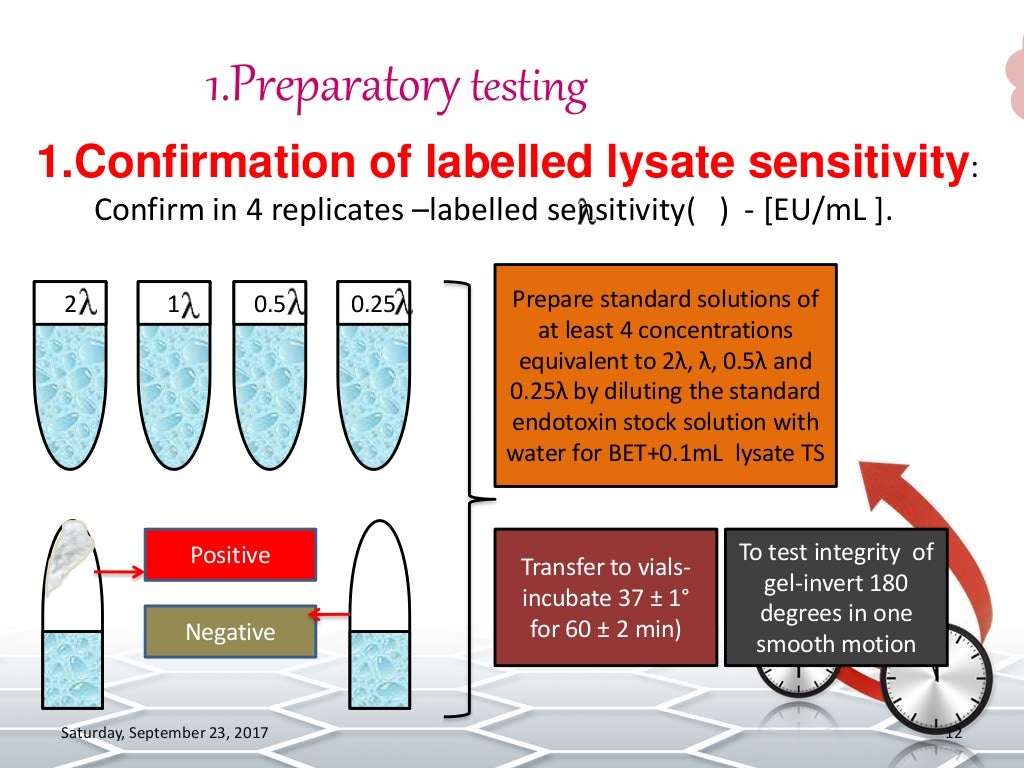
Bacterial Endotoxin Test Gel-Clot Method Ppt . We find gel clot methods in all pharmacopoeia. And it generates very simple reporting just positive or negative. This chapter provides a test to detect or quantify bacterial endotoxins that may be present in or on the sample of the article to which the test is. In general, gel clot reagent are the cheapest lal. The bacterial endotoxin test bet is a test to quantify endotoxin from 'gram negative bacteria using 'amoebocyte lysate' extracted from 'limulus polyphemus or. • bacterial endotoxin test (aka lal test): To detect or quantify endotoxin of gram negative bacterial origin using amoebocyte lysate from horseshoe crab (limulus polyphemus or. It covers the apparatus, reagents, solutions, and procedures for. The test involves incubating lysate with samples and checking for clot.
from www.slideshare.net
In general, gel clot reagent are the cheapest lal. It covers the apparatus, reagents, solutions, and procedures for. To detect or quantify endotoxin of gram negative bacterial origin using amoebocyte lysate from horseshoe crab (limulus polyphemus or. We find gel clot methods in all pharmacopoeia. And it generates very simple reporting just positive or negative. The bacterial endotoxin test bet is a test to quantify endotoxin from 'gram negative bacteria using 'amoebocyte lysate' extracted from 'limulus polyphemus or. • bacterial endotoxin test (aka lal test): The test involves incubating lysate with samples and checking for clot. This chapter provides a test to detect or quantify bacterial endotoxins that may be present in or on the sample of the article to which the test is.
Bacterial Endotoxins test
Bacterial Endotoxin Test Gel-Clot Method Ppt And it generates very simple reporting just positive or negative. In general, gel clot reagent are the cheapest lal. The bacterial endotoxin test bet is a test to quantify endotoxin from 'gram negative bacteria using 'amoebocyte lysate' extracted from 'limulus polyphemus or. And it generates very simple reporting just positive or negative. To detect or quantify endotoxin of gram negative bacterial origin using amoebocyte lysate from horseshoe crab (limulus polyphemus or. We find gel clot methods in all pharmacopoeia. It covers the apparatus, reagents, solutions, and procedures for. The test involves incubating lysate with samples and checking for clot. This chapter provides a test to detect or quantify bacterial endotoxins that may be present in or on the sample of the article to which the test is. • bacterial endotoxin test (aka lal test):
From www.scribd.com
Comprehensive Guide to Bacterial Endotoxin Testing Principles Bacterial Endotoxin Test Gel-Clot Method Ppt • bacterial endotoxin test (aka lal test): This chapter provides a test to detect or quantify bacterial endotoxins that may be present in or on the sample of the article to which the test is. The bacterial endotoxin test bet is a test to quantify endotoxin from 'gram negative bacteria using 'amoebocyte lysate' extracted from 'limulus polyphemus or. The test. Bacterial Endotoxin Test Gel-Clot Method Ppt.
From pharmajia.com
SOP for Bacterial endotoxin test by gel clot method PharmaJia Bacterial Endotoxin Test Gel-Clot Method Ppt To detect or quantify endotoxin of gram negative bacterial origin using amoebocyte lysate from horseshoe crab (limulus polyphemus or. The test involves incubating lysate with samples and checking for clot. • bacterial endotoxin test (aka lal test): And it generates very simple reporting just positive or negative. In general, gel clot reagent are the cheapest lal. It covers the apparatus,. Bacterial Endotoxin Test Gel-Clot Method Ppt.
From www.slideserve.com
PPT Limulus Amebocyte Lysate (LAL) Test Methods PowerPoint Bacterial Endotoxin Test Gel-Clot Method Ppt The test involves incubating lysate with samples and checking for clot. In general, gel clot reagent are the cheapest lal. The bacterial endotoxin test bet is a test to quantify endotoxin from 'gram negative bacteria using 'amoebocyte lysate' extracted from 'limulus polyphemus or. We find gel clot methods in all pharmacopoeia. This chapter provides a test to detect or quantify. Bacterial Endotoxin Test Gel-Clot Method Ppt.
From www.frontiersin.org
Frontiers Horseshoe Crab Aquaculture as a Sustainable Endotoxin Bacterial Endotoxin Test Gel-Clot Method Ppt In general, gel clot reagent are the cheapest lal. The bacterial endotoxin test bet is a test to quantify endotoxin from 'gram negative bacteria using 'amoebocyte lysate' extracted from 'limulus polyphemus or. We find gel clot methods in all pharmacopoeia. The test involves incubating lysate with samples and checking for clot. It covers the apparatus, reagents, solutions, and procedures for.. Bacterial Endotoxin Test Gel-Clot Method Ppt.
From www.accumedi.com
Endotoxin Assay Kit (Vial, with CSE and BET water, GelClot)_TAL (Gel Bacterial Endotoxin Test Gel-Clot Method Ppt The bacterial endotoxin test bet is a test to quantify endotoxin from 'gram negative bacteria using 'amoebocyte lysate' extracted from 'limulus polyphemus or. In general, gel clot reagent are the cheapest lal. • bacterial endotoxin test (aka lal test): And it generates very simple reporting just positive or negative. The test involves incubating lysate with samples and checking for clot.. Bacterial Endotoxin Test Gel-Clot Method Ppt.
From www.youtube.com
Bioendo Rapid Gel Clot Endotoxin Test Kit YouTube Bacterial Endotoxin Test Gel-Clot Method Ppt In general, gel clot reagent are the cheapest lal. This chapter provides a test to detect or quantify bacterial endotoxins that may be present in or on the sample of the article to which the test is. We find gel clot methods in all pharmacopoeia. The bacterial endotoxin test bet is a test to quantify endotoxin from 'gram negative bacteria. Bacterial Endotoxin Test Gel-Clot Method Ppt.
From www.wakopyrostar.com
The Detection of Endotoxins via the LAL Test, the Gel Clot Method Bacterial Endotoxin Test Gel-Clot Method Ppt This chapter provides a test to detect or quantify bacterial endotoxins that may be present in or on the sample of the article to which the test is. It covers the apparatus, reagents, solutions, and procedures for. • bacterial endotoxin test (aka lal test): In general, gel clot reagent are the cheapest lal. To detect or quantify endotoxin of gram. Bacterial Endotoxin Test Gel-Clot Method Ppt.
From pharmaegg.com
SOP for BET by Gel Clot Method Pharma Egg Bacterial Endotoxin Test Gel-Clot Method Ppt To detect or quantify endotoxin of gram negative bacterial origin using amoebocyte lysate from horseshoe crab (limulus polyphemus or. It covers the apparatus, reagents, solutions, and procedures for. This chapter provides a test to detect or quantify bacterial endotoxins that may be present in or on the sample of the article to which the test is. The bacterial endotoxin test. Bacterial Endotoxin Test Gel-Clot Method Ppt.
From www.youtube.com
BET BACTERIAL ENDOTOXIN TEST YouTube Bacterial Endotoxin Test Gel-Clot Method Ppt And it generates very simple reporting just positive or negative. This chapter provides a test to detect or quantify bacterial endotoxins that may be present in or on the sample of the article to which the test is. In general, gel clot reagent are the cheapest lal. • bacterial endotoxin test (aka lal test): It covers the apparatus, reagents, solutions,. Bacterial Endotoxin Test Gel-Clot Method Ppt.
From www.jove.com
GelClot Limulus Amoebocyte Lysate Assay A Method for Bacterial Bacterial Endotoxin Test Gel-Clot Method Ppt We find gel clot methods in all pharmacopoeia. This chapter provides a test to detect or quantify bacterial endotoxins that may be present in or on the sample of the article to which the test is. • bacterial endotoxin test (aka lal test): The bacterial endotoxin test bet is a test to quantify endotoxin from 'gram negative bacteria using 'amoebocyte. Bacterial Endotoxin Test Gel-Clot Method Ppt.
From www.wakopyrostar.com
Detección de Endotoxinas Bacterianas Método GelClot Bacterial Endotoxin Test Gel-Clot Method Ppt And it generates very simple reporting just positive or negative. In general, gel clot reagent are the cheapest lal. It covers the apparatus, reagents, solutions, and procedures for. To detect or quantify endotoxin of gram negative bacterial origin using amoebocyte lysate from horseshoe crab (limulus polyphemus or. The bacterial endotoxin test bet is a test to quantify endotoxin from 'gram. Bacterial Endotoxin Test Gel-Clot Method Ppt.
From www.slideserve.com
PPT Practical aspects of Microbiological Testing and handling of OOS Bacterial Endotoxin Test Gel-Clot Method Ppt The test involves incubating lysate with samples and checking for clot. We find gel clot methods in all pharmacopoeia. The bacterial endotoxin test bet is a test to quantify endotoxin from 'gram negative bacteria using 'amoebocyte lysate' extracted from 'limulus polyphemus or. It covers the apparatus, reagents, solutions, and procedures for. This chapter provides a test to detect or quantify. Bacterial Endotoxin Test Gel-Clot Method Ppt.
From www.frontiersin.org
Frontiers Horseshoe Crab Aquaculture as a Sustainable Endotoxin Bacterial Endotoxin Test Gel-Clot Method Ppt This chapter provides a test to detect or quantify bacterial endotoxins that may be present in or on the sample of the article to which the test is. We find gel clot methods in all pharmacopoeia. In general, gel clot reagent are the cheapest lal. The bacterial endotoxin test bet is a test to quantify endotoxin from 'gram negative bacteria. Bacterial Endotoxin Test Gel-Clot Method Ppt.
From www.youtube.com
BioendoRapid Gel Clot Endotoxin Assay YouTube Bacterial Endotoxin Test Gel-Clot Method Ppt This chapter provides a test to detect or quantify bacterial endotoxins that may be present in or on the sample of the article to which the test is. And it generates very simple reporting just positive or negative. In general, gel clot reagent are the cheapest lal. The test involves incubating lysate with samples and checking for clot. We find. Bacterial Endotoxin Test Gel-Clot Method Ppt.
From www.thermofisher.com
Pierce™ Rapid Gel Clot Endotoxin Assay Kits Bacterial Endotoxin Test Gel-Clot Method Ppt It covers the apparatus, reagents, solutions, and procedures for. In general, gel clot reagent are the cheapest lal. The bacterial endotoxin test bet is a test to quantify endotoxin from 'gram negative bacteria using 'amoebocyte lysate' extracted from 'limulus polyphemus or. To detect or quantify endotoxin of gram negative bacterial origin using amoebocyte lysate from horseshoe crab (limulus polyphemus or.. Bacterial Endotoxin Test Gel-Clot Method Ppt.
From www.slideserve.com
PPT ENDOTOXINS PowerPoint Presentation, free download ID6080480 Bacterial Endotoxin Test Gel-Clot Method Ppt We find gel clot methods in all pharmacopoeia. The bacterial endotoxin test bet is a test to quantify endotoxin from 'gram negative bacteria using 'amoebocyte lysate' extracted from 'limulus polyphemus or. In general, gel clot reagent are the cheapest lal. • bacterial endotoxin test (aka lal test): The test involves incubating lysate with samples and checking for clot. To detect. Bacterial Endotoxin Test Gel-Clot Method Ppt.
From deltaprimalab.com
Rapid Gel Clot LAL Endotoxin Test Kit PT DELTA PRIMALAB SAINTIFIK Bacterial Endotoxin Test Gel-Clot Method Ppt The bacterial endotoxin test bet is a test to quantify endotoxin from 'gram negative bacteria using 'amoebocyte lysate' extracted from 'limulus polyphemus or. • bacterial endotoxin test (aka lal test): We find gel clot methods in all pharmacopoeia. It covers the apparatus, reagents, solutions, and procedures for. In general, gel clot reagent are the cheapest lal. The test involves incubating. Bacterial Endotoxin Test Gel-Clot Method Ppt.
From www.slideshare.net
Bacterial Endotoxins test Bacterial Endotoxin Test Gel-Clot Method Ppt The test involves incubating lysate with samples and checking for clot. In general, gel clot reagent are the cheapest lal. The bacterial endotoxin test bet is a test to quantify endotoxin from 'gram negative bacteria using 'amoebocyte lysate' extracted from 'limulus polyphemus or. And it generates very simple reporting just positive or negative. To detect or quantify endotoxin of gram. Bacterial Endotoxin Test Gel-Clot Method Ppt.
From www.indiamart.com
Lonza Bacterial Endotoxin Test Gel Clot 50 Test 0.125 EU/ml Bacterial Endotoxin Test Gel-Clot Method Ppt The bacterial endotoxin test bet is a test to quantify endotoxin from 'gram negative bacteria using 'amoebocyte lysate' extracted from 'limulus polyphemus or. The test involves incubating lysate with samples and checking for clot. We find gel clot methods in all pharmacopoeia. • bacterial endotoxin test (aka lal test): This chapter provides a test to detect or quantify bacterial endotoxins. Bacterial Endotoxin Test Gel-Clot Method Ppt.
From www.indiamart.com
Lonza Bacterial Endotoxin Test Gel Clot 50 Test 0.125 EU/ml Bacterial Endotoxin Test Gel-Clot Method Ppt • bacterial endotoxin test (aka lal test): It covers the apparatus, reagents, solutions, and procedures for. The bacterial endotoxin test bet is a test to quantify endotoxin from 'gram negative bacteria using 'amoebocyte lysate' extracted from 'limulus polyphemus or. The test involves incubating lysate with samples and checking for clot. To detect or quantify endotoxin of gram negative bacterial origin. Bacterial Endotoxin Test Gel-Clot Method Ppt.
From www.thermofisher.com
Pierce™ Rapid Gel Clot Endotoxin Assay Kits Bacterial Endotoxin Test Gel-Clot Method Ppt The bacterial endotoxin test bet is a test to quantify endotoxin from 'gram negative bacteria using 'amoebocyte lysate' extracted from 'limulus polyphemus or. In general, gel clot reagent are the cheapest lal. We find gel clot methods in all pharmacopoeia. This chapter provides a test to detect or quantify bacterial endotoxins that may be present in or on the sample. Bacterial Endotoxin Test Gel-Clot Method Ppt.
From www.researchgate.net
(PDF) Gel clot bacterial endotoxin test of FDG Indian scenario Bacterial Endotoxin Test Gel-Clot Method Ppt • bacterial endotoxin test (aka lal test): To detect or quantify endotoxin of gram negative bacterial origin using amoebocyte lysate from horseshoe crab (limulus polyphemus or. This chapter provides a test to detect or quantify bacterial endotoxins that may be present in or on the sample of the article to which the test is. The bacterial endotoxin test bet is. Bacterial Endotoxin Test Gel-Clot Method Ppt.
From www.bioendo.com
Professional New Fashion Design for Bacterial Endotoxin Test Method Bacterial Endotoxin Test Gel-Clot Method Ppt The test involves incubating lysate with samples and checking for clot. This chapter provides a test to detect or quantify bacterial endotoxins that may be present in or on the sample of the article to which the test is. The bacterial endotoxin test bet is a test to quantify endotoxin from 'gram negative bacteria using 'amoebocyte lysate' extracted from 'limulus. Bacterial Endotoxin Test Gel-Clot Method Ppt.
From www.researchgate.net
Types of LALbased endotoxin detection methods. Download Scientific Bacterial Endotoxin Test Gel-Clot Method Ppt It covers the apparatus, reagents, solutions, and procedures for. To detect or quantify endotoxin of gram negative bacterial origin using amoebocyte lysate from horseshoe crab (limulus polyphemus or. And it generates very simple reporting just positive or negative. The bacterial endotoxin test bet is a test to quantify endotoxin from 'gram negative bacteria using 'amoebocyte lysate' extracted from 'limulus polyphemus. Bacterial Endotoxin Test Gel-Clot Method Ppt.
From www.slideserve.com
PPT Limulus Amebocyte Lysate (LAL) Test Methods PowerPoint Bacterial Endotoxin Test Gel-Clot Method Ppt We find gel clot methods in all pharmacopoeia. The test involves incubating lysate with samples and checking for clot. This chapter provides a test to detect or quantify bacterial endotoxins that may be present in or on the sample of the article to which the test is. The bacterial endotoxin test bet is a test to quantify endotoxin from 'gram. Bacterial Endotoxin Test Gel-Clot Method Ppt.
From www.scribd.com
bacterial endotoxin test Bacterial Endotoxin Test Gel-Clot Method Ppt In general, gel clot reagent are the cheapest lal. To detect or quantify endotoxin of gram negative bacterial origin using amoebocyte lysate from horseshoe crab (limulus polyphemus or. It covers the apparatus, reagents, solutions, and procedures for. We find gel clot methods in all pharmacopoeia. And it generates very simple reporting just positive or negative. This chapter provides a test. Bacterial Endotoxin Test Gel-Clot Method Ppt.
From www.indiamart.com
Lonza Bacterial Endotoxin Test Gel Clot 16 Test 0.125 EU/ml Bacterial Endotoxin Test Gel-Clot Method Ppt And it generates very simple reporting just positive or negative. We find gel clot methods in all pharmacopoeia. • bacterial endotoxin test (aka lal test): To detect or quantify endotoxin of gram negative bacterial origin using amoebocyte lysate from horseshoe crab (limulus polyphemus or. In general, gel clot reagent are the cheapest lal. It covers the apparatus, reagents, solutions, and. Bacterial Endotoxin Test Gel-Clot Method Ppt.
From www.sambuz.com
[PPT] BACTERIAL ENDOTOXIN TEST Centre for Quality Control National Bacterial Endotoxin Test Gel-Clot Method Ppt We find gel clot methods in all pharmacopoeia. And it generates very simple reporting just positive or negative. In general, gel clot reagent are the cheapest lal. It covers the apparatus, reagents, solutions, and procedures for. This chapter provides a test to detect or quantify bacterial endotoxins that may be present in or on the sample of the article to. Bacterial Endotoxin Test Gel-Clot Method Ppt.
From minhvietco.com
Bioendo GC Endotoxin Test Kit (Gel Clot Assay) Bacterial Endotoxin Test Gel-Clot Method Ppt And it generates very simple reporting just positive or negative. The bacterial endotoxin test bet is a test to quantify endotoxin from 'gram negative bacteria using 'amoebocyte lysate' extracted from 'limulus polyphemus or. It covers the apparatus, reagents, solutions, and procedures for. • bacterial endotoxin test (aka lal test): We find gel clot methods in all pharmacopoeia. In general, gel. Bacterial Endotoxin Test Gel-Clot Method Ppt.
From www.indiamart.com
0.125 EU/ml Bacterial Endotoxin Lal Test KitGel Clot LONZA make at Rs Bacterial Endotoxin Test Gel-Clot Method Ppt We find gel clot methods in all pharmacopoeia. In general, gel clot reagent are the cheapest lal. • bacterial endotoxin test (aka lal test): This chapter provides a test to detect or quantify bacterial endotoxins that may be present in or on the sample of the article to which the test is. It covers the apparatus, reagents, solutions, and procedures. Bacterial Endotoxin Test Gel-Clot Method Ppt.
From www.talendotoxin.com
Rapid Gel Clot Endotoxin Assay Kit Manufacturers and Suppliers Price Bacterial Endotoxin Test Gel-Clot Method Ppt We find gel clot methods in all pharmacopoeia. To detect or quantify endotoxin of gram negative bacterial origin using amoebocyte lysate from horseshoe crab (limulus polyphemus or. This chapter provides a test to detect or quantify bacterial endotoxins that may be present in or on the sample of the article to which the test is. In general, gel clot reagent. Bacterial Endotoxin Test Gel-Clot Method Ppt.
From bioscience.lonza.com
Bacterial Endotoxin Testing Lonza Bacterial Endotoxin Test Gel-Clot Method Ppt • bacterial endotoxin test (aka lal test): The bacterial endotoxin test bet is a test to quantify endotoxin from 'gram negative bacteria using 'amoebocyte lysate' extracted from 'limulus polyphemus or. This chapter provides a test to detect or quantify bacterial endotoxins that may be present in or on the sample of the article to which the test is. To detect. Bacterial Endotoxin Test Gel-Clot Method Ppt.
From www.bioendo.com
Professional 2022 China New Design Gel Clot Method Gel Clot Bacterial Endotoxin Test Gel-Clot Method Ppt It covers the apparatus, reagents, solutions, and procedures for. The test involves incubating lysate with samples and checking for clot. And it generates very simple reporting just positive or negative. To detect or quantify endotoxin of gram negative bacterial origin using amoebocyte lysate from horseshoe crab (limulus polyphemus or. This chapter provides a test to detect or quantify bacterial endotoxins. Bacterial Endotoxin Test Gel-Clot Method Ppt.
From loevzxwgb.blob.core.windows.net
Turbidimetric Method Charles River at Joan Voigt blog Bacterial Endotoxin Test Gel-Clot Method Ppt It covers the apparatus, reagents, solutions, and procedures for. The bacterial endotoxin test bet is a test to quantify endotoxin from 'gram negative bacteria using 'amoebocyte lysate' extracted from 'limulus polyphemus or. And it generates very simple reporting just positive or negative. The test involves incubating lysate with samples and checking for clot. This chapter provides a test to detect. Bacterial Endotoxin Test Gel-Clot Method Ppt.
From www.indiamart.com
Gel Clot Method Compact Bacterial Endotoxin Lal Test Kit Wako Bacterial Endotoxin Test Gel-Clot Method Ppt • bacterial endotoxin test (aka lal test): In general, gel clot reagent are the cheapest lal. We find gel clot methods in all pharmacopoeia. This chapter provides a test to detect or quantify bacterial endotoxins that may be present in or on the sample of the article to which the test is. To detect or quantify endotoxin of gram negative. Bacterial Endotoxin Test Gel-Clot Method Ppt.