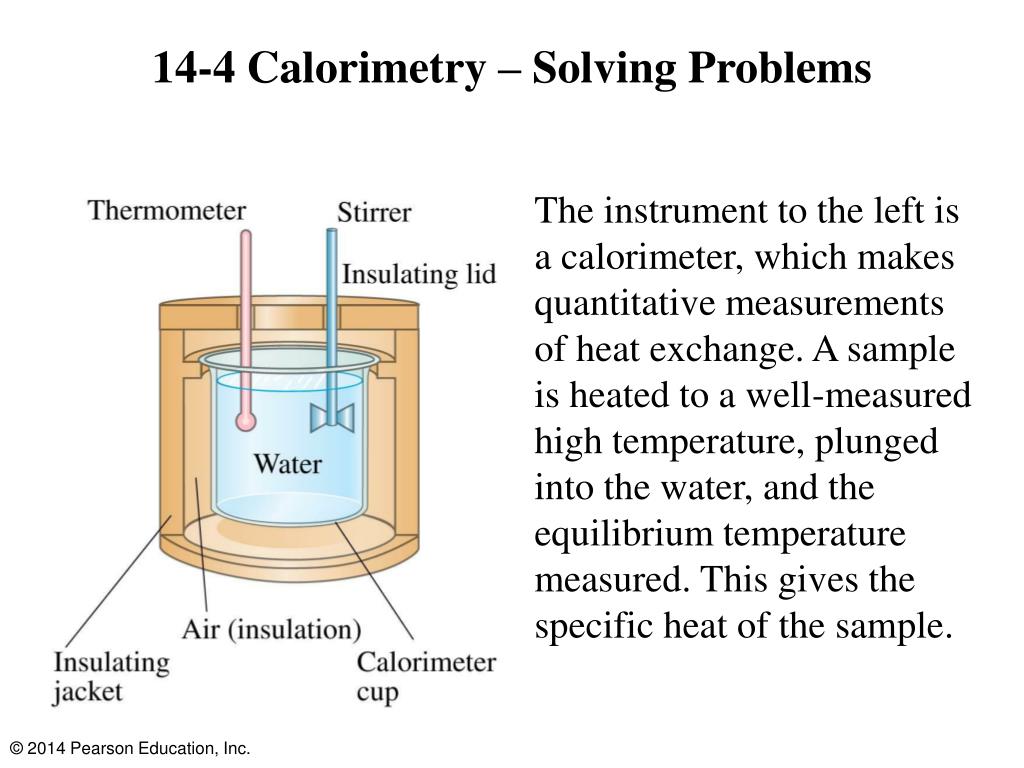
What Basic Assumptions Are Made In Calorimetry Experiments . All the energy lost or gained by the chemical system is gained or lost (respectively) by the calorimeter; A calorimeter is a device used to determine heat flow during a. In the laboratory, heat flow is measured in an apparatus called a calorimeter. Learn how to measure the heat energy transferred during combustion, displacement, dissolving and neutralisation reactions using calorimetry. The apparatus needed to carry out an enthalpy of reaction in solution calorimetry experiment is shown in the previous section 5.1.3. We can experimentally determine the relative amounts of energy released by a fuel. There are two types of calorimetry experiments you need to know: Calorimetry measurements are important in understanding the heat transferred in reactions involving everything from microscopic proteins. We do this using simple calorimetry. Enthalpy changes of reactions in solution, e.g.
from www.slideserve.com
Enthalpy changes of reactions in solution, e.g. We do this using simple calorimetry. We can experimentally determine the relative amounts of energy released by a fuel. The apparatus needed to carry out an enthalpy of reaction in solution calorimetry experiment is shown in the previous section 5.1.3. In the laboratory, heat flow is measured in an apparatus called a calorimeter. All the energy lost or gained by the chemical system is gained or lost (respectively) by the calorimeter; Learn how to measure the heat energy transferred during combustion, displacement, dissolving and neutralisation reactions using calorimetry. There are two types of calorimetry experiments you need to know: A calorimeter is a device used to determine heat flow during a. Calorimetry measurements are important in understanding the heat transferred in reactions involving everything from microscopic proteins.
PPT Chapter 14 Heat PowerPoint Presentation, free download ID6832402
What Basic Assumptions Are Made In Calorimetry Experiments A calorimeter is a device used to determine heat flow during a. We do this using simple calorimetry. All the energy lost or gained by the chemical system is gained or lost (respectively) by the calorimeter; Calorimetry measurements are important in understanding the heat transferred in reactions involving everything from microscopic proteins. In the laboratory, heat flow is measured in an apparatus called a calorimeter. The apparatus needed to carry out an enthalpy of reaction in solution calorimetry experiment is shown in the previous section 5.1.3. There are two types of calorimetry experiments you need to know: A calorimeter is a device used to determine heat flow during a. Enthalpy changes of reactions in solution, e.g. Learn how to measure the heat energy transferred during combustion, displacement, dissolving and neutralisation reactions using calorimetry. We can experimentally determine the relative amounts of energy released by a fuel.
From ar.inspiredpencil.com
Simple Bomb Calorimeter What Basic Assumptions Are Made In Calorimetry Experiments A calorimeter is a device used to determine heat flow during a. We do this using simple calorimetry. The apparatus needed to carry out an enthalpy of reaction in solution calorimetry experiment is shown in the previous section 5.1.3. There are two types of calorimetry experiments you need to know: We can experimentally determine the relative amounts of energy released. What Basic Assumptions Are Made In Calorimetry Experiments.
From joicpmtos.blob.core.windows.net
Calorimeter In Particle Physics at Krystina Moberg blog What Basic Assumptions Are Made In Calorimetry Experiments Enthalpy changes of reactions in solution, e.g. There are two types of calorimetry experiments you need to know: In the laboratory, heat flow is measured in an apparatus called a calorimeter. All the energy lost or gained by the chemical system is gained or lost (respectively) by the calorimeter; Learn how to measure the heat energy transferred during combustion, displacement,. What Basic Assumptions Are Made In Calorimetry Experiments.
From www.nagwa.com
Question Video Determining the Correct Formula to Use in Order to What Basic Assumptions Are Made In Calorimetry Experiments In the laboratory, heat flow is measured in an apparatus called a calorimeter. We do this using simple calorimetry. The apparatus needed to carry out an enthalpy of reaction in solution calorimetry experiment is shown in the previous section 5.1.3. Enthalpy changes of reactions in solution, e.g. Calorimetry measurements are important in understanding the heat transferred in reactions involving everything. What Basic Assumptions Are Made In Calorimetry Experiments.
From klarxnzah.blob.core.windows.net
Calorimetry Experiment With Different Metals at David Lytton blog What Basic Assumptions Are Made In Calorimetry Experiments All the energy lost or gained by the chemical system is gained or lost (respectively) by the calorimeter; Enthalpy changes of reactions in solution, e.g. There are two types of calorimetry experiments you need to know: Calorimetry measurements are important in understanding the heat transferred in reactions involving everything from microscopic proteins. Learn how to measure the heat energy transferred. What Basic Assumptions Are Made In Calorimetry Experiments.
From www.slideshare.net
Bomb calorimeter experiment What Basic Assumptions Are Made In Calorimetry Experiments All the energy lost or gained by the chemical system is gained or lost (respectively) by the calorimeter; We do this using simple calorimetry. In the laboratory, heat flow is measured in an apparatus called a calorimeter. The apparatus needed to carry out an enthalpy of reaction in solution calorimetry experiment is shown in the previous section 5.1.3. There are. What Basic Assumptions Are Made In Calorimetry Experiments.
From printablecampusreises.z21.web.core.windows.net
Solving A Basic Calorimetry Problem What Basic Assumptions Are Made In Calorimetry Experiments Learn how to measure the heat energy transferred during combustion, displacement, dissolving and neutralisation reactions using calorimetry. Calorimetry measurements are important in understanding the heat transferred in reactions involving everything from microscopic proteins. We do this using simple calorimetry. In the laboratory, heat flow is measured in an apparatus called a calorimeter. We can experimentally determine the relative amounts of. What Basic Assumptions Are Made In Calorimetry Experiments.
From socratic.org
A student conducting a calorimetry investigation determines a negative What Basic Assumptions Are Made In Calorimetry Experiments A calorimeter is a device used to determine heat flow during a. Learn how to measure the heat energy transferred during combustion, displacement, dissolving and neutralisation reactions using calorimetry. There are two types of calorimetry experiments you need to know: Enthalpy changes of reactions in solution, e.g. We do this using simple calorimetry. We can experimentally determine the relative amounts. What Basic Assumptions Are Made In Calorimetry Experiments.
From chem.libretexts.org
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts What Basic Assumptions Are Made In Calorimetry Experiments We can experimentally determine the relative amounts of energy released by a fuel. Learn how to measure the heat energy transferred during combustion, displacement, dissolving and neutralisation reactions using calorimetry. Calorimetry measurements are important in understanding the heat transferred in reactions involving everything from microscopic proteins. Enthalpy changes of reactions in solution, e.g. A calorimeter is a device used to. What Basic Assumptions Are Made In Calorimetry Experiments.
From slideplayer.com
A “Calorimeter”. Calorimetry Calculations When analyzing data obtained What Basic Assumptions Are Made In Calorimetry Experiments Learn how to measure the heat energy transferred during combustion, displacement, dissolving and neutralisation reactions using calorimetry. A calorimeter is a device used to determine heat flow during a. In the laboratory, heat flow is measured in an apparatus called a calorimeter. We do this using simple calorimetry. We can experimentally determine the relative amounts of energy released by a. What Basic Assumptions Are Made In Calorimetry Experiments.
From klarxnzah.blob.core.windows.net
Calorimetry Experiment With Different Metals at David Lytton blog What Basic Assumptions Are Made In Calorimetry Experiments Learn how to measure the heat energy transferred during combustion, displacement, dissolving and neutralisation reactions using calorimetry. We do this using simple calorimetry. There are two types of calorimetry experiments you need to know: Enthalpy changes of reactions in solution, e.g. All the energy lost or gained by the chemical system is gained or lost (respectively) by the calorimeter; A. What Basic Assumptions Are Made In Calorimetry Experiments.
From loerzgmcm.blob.core.windows.net
How To Make A Homemade Calorimeter at Norma Mercer blog What Basic Assumptions Are Made In Calorimetry Experiments Learn how to measure the heat energy transferred during combustion, displacement, dissolving and neutralisation reactions using calorimetry. We can experimentally determine the relative amounts of energy released by a fuel. There are two types of calorimetry experiments you need to know: Calorimetry measurements are important in understanding the heat transferred in reactions involving everything from microscopic proteins. All the energy. What Basic Assumptions Are Made In Calorimetry Experiments.
From chemistrytalk.org
Calorimetry ChemTalk What Basic Assumptions Are Made In Calorimetry Experiments The apparatus needed to carry out an enthalpy of reaction in solution calorimetry experiment is shown in the previous section 5.1.3. All the energy lost or gained by the chemical system is gained or lost (respectively) by the calorimeter; A calorimeter is a device used to determine heat flow during a. In the laboratory, heat flow is measured in an. What Basic Assumptions Are Made In Calorimetry Experiments.
From exonwzknq.blob.core.windows.net
Calorimetry Experiment Gcse Chemistry at Sanders blog What Basic Assumptions Are Made In Calorimetry Experiments The apparatus needed to carry out an enthalpy of reaction in solution calorimetry experiment is shown in the previous section 5.1.3. Learn how to measure the heat energy transferred during combustion, displacement, dissolving and neutralisation reactions using calorimetry. In the laboratory, heat flow is measured in an apparatus called a calorimeter. We do this using simple calorimetry. All the energy. What Basic Assumptions Are Made In Calorimetry Experiments.
From joiddcrwa.blob.core.windows.net
What Is Calorimetry Example at Donovan blog What Basic Assumptions Are Made In Calorimetry Experiments Enthalpy changes of reactions in solution, e.g. Calorimetry measurements are important in understanding the heat transferred in reactions involving everything from microscopic proteins. We do this using simple calorimetry. The apparatus needed to carry out an enthalpy of reaction in solution calorimetry experiment is shown in the previous section 5.1.3. We can experimentally determine the relative amounts of energy released. What Basic Assumptions Are Made In Calorimetry Experiments.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment What Basic Assumptions Are Made In Calorimetry Experiments Enthalpy changes of reactions in solution, e.g. We can experimentally determine the relative amounts of energy released by a fuel. All the energy lost or gained by the chemical system is gained or lost (respectively) by the calorimeter; The apparatus needed to carry out an enthalpy of reaction in solution calorimetry experiment is shown in the previous section 5.1.3. Calorimetry. What Basic Assumptions Are Made In Calorimetry Experiments.
From www.slideserve.com
PPT Energy in food PowerPoint Presentation, free download ID5830775 What Basic Assumptions Are Made In Calorimetry Experiments There are two types of calorimetry experiments you need to know: Enthalpy changes of reactions in solution, e.g. Learn how to measure the heat energy transferred during combustion, displacement, dissolving and neutralisation reactions using calorimetry. We do this using simple calorimetry. In the laboratory, heat flow is measured in an apparatus called a calorimeter. Calorimetry measurements are important in understanding. What Basic Assumptions Are Made In Calorimetry Experiments.
From www.savemyexams.com
Calorimetry Experiments SL IB Chemistry Revision Notes 2025 Save My What Basic Assumptions Are Made In Calorimetry Experiments All the energy lost or gained by the chemical system is gained or lost (respectively) by the calorimeter; A calorimeter is a device used to determine heat flow during a. Calorimetry measurements are important in understanding the heat transferred in reactions involving everything from microscopic proteins. The apparatus needed to carry out an enthalpy of reaction in solution calorimetry experiment. What Basic Assumptions Are Made In Calorimetry Experiments.
From www.linstitute.net
IB DP Chemistry HL复习笔记5.1.4 Calorimetry Experiments翰林国际教育 What Basic Assumptions Are Made In Calorimetry Experiments We do this using simple calorimetry. All the energy lost or gained by the chemical system is gained or lost (respectively) by the calorimeter; The apparatus needed to carry out an enthalpy of reaction in solution calorimetry experiment is shown in the previous section 5.1.3. A calorimeter is a device used to determine heat flow during a. Learn how to. What Basic Assumptions Are Made In Calorimetry Experiments.
From www.youtube.com
Constructing a Calorimeter YouTube What Basic Assumptions Are Made In Calorimetry Experiments The apparatus needed to carry out an enthalpy of reaction in solution calorimetry experiment is shown in the previous section 5.1.3. There are two types of calorimetry experiments you need to know: Learn how to measure the heat energy transferred during combustion, displacement, dissolving and neutralisation reactions using calorimetry. In the laboratory, heat flow is measured in an apparatus called. What Basic Assumptions Are Made In Calorimetry Experiments.
From www.numerade.com
SOLVED Lab Calorimetry of Salt Solution Procedure Measure NaOH on What Basic Assumptions Are Made In Calorimetry Experiments There are two types of calorimetry experiments you need to know: Enthalpy changes of reactions in solution, e.g. All the energy lost or gained by the chemical system is gained or lost (respectively) by the calorimeter; We can experimentally determine the relative amounts of energy released by a fuel. Learn how to measure the heat energy transferred during combustion, displacement,. What Basic Assumptions Are Made In Calorimetry Experiments.
From klayjstec.blob.core.windows.net
How Calorimeter Function at Geneva Katzman blog What Basic Assumptions Are Made In Calorimetry Experiments All the energy lost or gained by the chemical system is gained or lost (respectively) by the calorimeter; Enthalpy changes of reactions in solution, e.g. We can experimentally determine the relative amounts of energy released by a fuel. There are two types of calorimetry experiments you need to know: Learn how to measure the heat energy transferred during combustion, displacement,. What Basic Assumptions Are Made In Calorimetry Experiments.
From www.pinterest.com
Calorimetry Bomb Calorimeter Experiment Science fair, Homeschool and What Basic Assumptions Are Made In Calorimetry Experiments We do this using simple calorimetry. The apparatus needed to carry out an enthalpy of reaction in solution calorimetry experiment is shown in the previous section 5.1.3. Calorimetry measurements are important in understanding the heat transferred in reactions involving everything from microscopic proteins. Learn how to measure the heat energy transferred during combustion, displacement, dissolving and neutralisation reactions using calorimetry.. What Basic Assumptions Are Made In Calorimetry Experiments.
From cepfukqw.blob.core.windows.net
When Is Calorimetry Used In Real Life at John Nelson blog What Basic Assumptions Are Made In Calorimetry Experiments In the laboratory, heat flow is measured in an apparatus called a calorimeter. A calorimeter is a device used to determine heat flow during a. We do this using simple calorimetry. We can experimentally determine the relative amounts of energy released by a fuel. Learn how to measure the heat energy transferred during combustion, displacement, dissolving and neutralisation reactions using. What Basic Assumptions Are Made In Calorimetry Experiments.
From ar.inspiredpencil.com
Simple Bomb Calorimeter What Basic Assumptions Are Made In Calorimetry Experiments We do this using simple calorimetry. In the laboratory, heat flow is measured in an apparatus called a calorimeter. The apparatus needed to carry out an enthalpy of reaction in solution calorimetry experiment is shown in the previous section 5.1.3. Enthalpy changes of reactions in solution, e.g. All the energy lost or gained by the chemical system is gained or. What Basic Assumptions Are Made In Calorimetry Experiments.
From klaqayslh.blob.core.windows.net
Calorimeter Experiment Sources Of Error at Rudy Reichman blog What Basic Assumptions Are Made In Calorimetry Experiments We can experimentally determine the relative amounts of energy released by a fuel. In the laboratory, heat flow is measured in an apparatus called a calorimeter. A calorimeter is a device used to determine heat flow during a. The apparatus needed to carry out an enthalpy of reaction in solution calorimetry experiment is shown in the previous section 5.1.3. Learn. What Basic Assumptions Are Made In Calorimetry Experiments.
From www.scienceabc.com
How Do We Calculate How Many Carbs Are In The Food? What Basic Assumptions Are Made In Calorimetry Experiments Learn how to measure the heat energy transferred during combustion, displacement, dissolving and neutralisation reactions using calorimetry. Enthalpy changes of reactions in solution, e.g. We do this using simple calorimetry. In the laboratory, heat flow is measured in an apparatus called a calorimeter. We can experimentally determine the relative amounts of energy released by a fuel. The apparatus needed to. What Basic Assumptions Are Made In Calorimetry Experiments.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle What Basic Assumptions Are Made In Calorimetry Experiments Learn how to measure the heat energy transferred during combustion, displacement, dissolving and neutralisation reactions using calorimetry. The apparatus needed to carry out an enthalpy of reaction in solution calorimetry experiment is shown in the previous section 5.1.3. In the laboratory, heat flow is measured in an apparatus called a calorimeter. We can experimentally determine the relative amounts of energy. What Basic Assumptions Are Made In Calorimetry Experiments.
From www.baamboozle.com
Heat Gain and Heat Loss Baamboozle Baamboozle The Most Fun What Basic Assumptions Are Made In Calorimetry Experiments Calorimetry measurements are important in understanding the heat transferred in reactions involving everything from microscopic proteins. All the energy lost or gained by the chemical system is gained or lost (respectively) by the calorimeter; We do this using simple calorimetry. We can experimentally determine the relative amounts of energy released by a fuel. Learn how to measure the heat energy. What Basic Assumptions Are Made In Calorimetry Experiments.
From loerzgmcm.blob.core.windows.net
How To Make A Homemade Calorimeter at Norma Mercer blog What Basic Assumptions Are Made In Calorimetry Experiments We do this using simple calorimetry. All the energy lost or gained by the chemical system is gained or lost (respectively) by the calorimeter; Calorimetry measurements are important in understanding the heat transferred in reactions involving everything from microscopic proteins. Enthalpy changes of reactions in solution, e.g. In the laboratory, heat flow is measured in an apparatus called a calorimeter.. What Basic Assumptions Are Made In Calorimetry Experiments.
From www.slideserve.com
PPT Chapter 12 “Heat in Chemical Reactions” PowerPoint Presentation What Basic Assumptions Are Made In Calorimetry Experiments Enthalpy changes of reactions in solution, e.g. In the laboratory, heat flow is measured in an apparatus called a calorimeter. There are two types of calorimetry experiments you need to know: A calorimeter is a device used to determine heat flow during a. The apparatus needed to carry out an enthalpy of reaction in solution calorimetry experiment is shown in. What Basic Assumptions Are Made In Calorimetry Experiments.
From klaqayslh.blob.core.windows.net
Calorimeter Experiment Sources Of Error at Rudy Reichman blog What Basic Assumptions Are Made In Calorimetry Experiments Learn how to measure the heat energy transferred during combustion, displacement, dissolving and neutralisation reactions using calorimetry. A calorimeter is a device used to determine heat flow during a. We can experimentally determine the relative amounts of energy released by a fuel. There are two types of calorimetry experiments you need to know: All the energy lost or gained by. What Basic Assumptions Are Made In Calorimetry Experiments.
From exollkmhz.blob.core.windows.net
Define Adiabatic Calorimeter at Alma Martin blog What Basic Assumptions Are Made In Calorimetry Experiments There are two types of calorimetry experiments you need to know: The apparatus needed to carry out an enthalpy of reaction in solution calorimetry experiment is shown in the previous section 5.1.3. We do this using simple calorimetry. We can experimentally determine the relative amounts of energy released by a fuel. All the energy lost or gained by the chemical. What Basic Assumptions Are Made In Calorimetry Experiments.
From www.nagwa.com
Question Video Identifying the Factor That Does Not Need to Be Held What Basic Assumptions Are Made In Calorimetry Experiments In the laboratory, heat flow is measured in an apparatus called a calorimeter. The apparatus needed to carry out an enthalpy of reaction in solution calorimetry experiment is shown in the previous section 5.1.3. A calorimeter is a device used to determine heat flow during a. All the energy lost or gained by the chemical system is gained or lost. What Basic Assumptions Are Made In Calorimetry Experiments.
From www.youtube.com
Calorimetry, Bomb Calorimetry, Constant Pressure Calorimetry FULL What Basic Assumptions Are Made In Calorimetry Experiments The apparatus needed to carry out an enthalpy of reaction in solution calorimetry experiment is shown in the previous section 5.1.3. Learn how to measure the heat energy transferred during combustion, displacement, dissolving and neutralisation reactions using calorimetry. Enthalpy changes of reactions in solution, e.g. Calorimetry measurements are important in understanding the heat transferred in reactions involving everything from microscopic. What Basic Assumptions Are Made In Calorimetry Experiments.
From www.slideserve.com
PPT Chapter 14 Heat PowerPoint Presentation, free download ID6832402 What Basic Assumptions Are Made In Calorimetry Experiments We can experimentally determine the relative amounts of energy released by a fuel. Learn how to measure the heat energy transferred during combustion, displacement, dissolving and neutralisation reactions using calorimetry. There are two types of calorimetry experiments you need to know: All the energy lost or gained by the chemical system is gained or lost (respectively) by the calorimeter; In. What Basic Assumptions Are Made In Calorimetry Experiments.