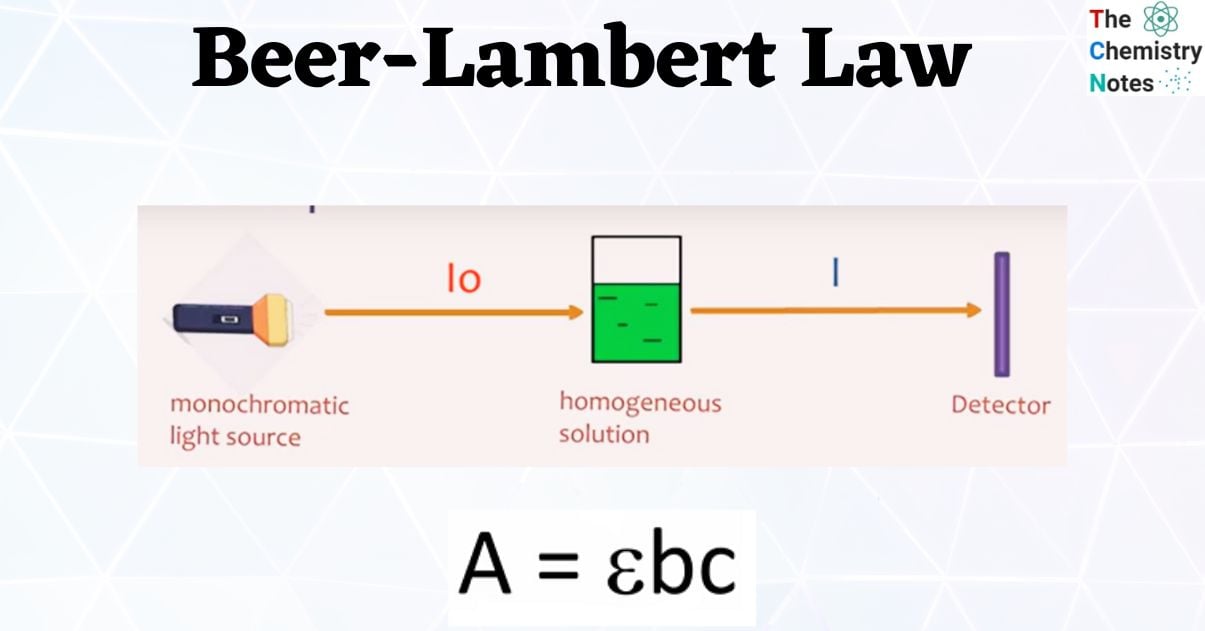
Beer Lambert Law Lab . “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light. It gives a relationship between the concentration of a solution and the attenuation of light as it. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). Determine order of reaction by measuring the. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law).
from scienceinfo.com
It gives a relationship between the concentration of a solution and the attenuation of light as it. Determine order of reaction by measuring the. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer.
BeerLambert Law Statement, Derivation, Applications, Limitations
Beer Lambert Law Lab “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light. “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light. Determine order of reaction by measuring the. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. It gives a relationship between the concentration of a solution and the attenuation of light as it. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\).
From www.researchgate.net
1 a Schematic representation for BeerLambert law for the measurement Beer Lambert Law Lab Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. It gives a relationship between the concentration of a solution and the attenuation of light as it. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Calculate the concentration of a dilute aqueous solute using absorbance. Beer Lambert Law Lab.
From scienceinfo.com
BeerLambert Law Statement, Derivation, Applications, Limitations Beer Lambert Law Lab Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light. Determine order of reaction by measuring the. Consider monochromatic light of a given intensity incident on a sample, as shown in. Beer Lambert Law Lab.
From studylib.net
Beer's Law Lab Beer Lambert Law Lab Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Calculate the concentration of a. Beer Lambert Law Lab.
From www.studocu.com
BeerLambert Law BeerLambert Law Lab Report BeerLambert Law Beer Lambert Law Lab It gives a relationship between the concentration of a solution and the attenuation of light as it. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Determine order of reaction by measuring the. “the thicker the. Beer Lambert Law Lab.
From www.vernier.com
Determining the Concentration of a Solution Beer's Law > Experiment 17 Beer Lambert Law Lab Determine order of reaction by measuring the. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light. Explore beer's law by creating colorful solutions and measuring light absorption. Beer Lambert Law Lab.
From studylib.net
The BeerLambert Law Beer Lambert Law Lab “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The amount. Beer Lambert Law Lab.
From goformative.com
Beer's Law Simulation for AP Chemistry Carson Dobrin Library Formative Beer Lambert Law Lab In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. It gives a relationship between the concentration of a solution and the attenuation of light as it. Determine order of reaction by measuring the. “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute. Beer Lambert Law Lab.
From www.geeksforgeeks.org
BeerLambert Law Statement, Formula, Equation & Derivation Beer Lambert Law Lab Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. The amount of light that a species absorbs in a spectroscopic transition can be. Beer Lambert Law Lab.
From studylib.net
Casestudy The BeerLambert Law and Spectrophotometry Learning objectives Beer Lambert Law Lab Determine order of reaction by measuring the. It gives a relationship between the concentration of a solution and the attenuation of light as it. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). “the thicker the. Beer Lambert Law Lab.
From www.youtube.com
Beer Lambert Law, Molar Extinction Coefficient, Spectrophotometry YouTube Beer Lambert Law Lab “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. It gives a relationship between the concentration of a solution and the attenuation of light as it. Explore. Beer Lambert Law Lab.
From www.youtube.com
Analytical Instrumentation Tutorial 2 Beer Lambert Law YouTube Beer Lambert Law Lab Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. It gives a relationship between the concentration of a solution and the attenuation of light as it. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Calculate the concentration of a dilute aqueous solute using absorbance. Beer Lambert Law Lab.
From www.slideserve.com
PPT SPECTROPHOTOMETRY PowerPoint Presentation, free download ID5761859 Beer Lambert Law Lab In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light. Calculate. Beer Lambert Law Lab.
From studylib.net
Beer Lambert Law Beer Lambert Law Lab Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to. Beer Lambert Law Lab.
From www.youtube.com
Introduction to UVVis Spectroscopy 03 BeerLambert Law YouTube Beer Lambert Law Lab Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). Determine order of reaction by measuring the. “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light. The amount of light that a species absorbs in a spectroscopic. Beer Lambert Law Lab.
From slidetodoc.com
Part 2 9 Electronic Transitions Outline Absorption spectroscopy Beer Lambert Law Lab The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light. Consider monochromatic light of a given intensity incident on a sample, as. Beer Lambert Law Lab.
From www.edinst.com
Beer Lambert Law Transmittance & Absorbance Edinburgh Instruments Beer Lambert Law Lab Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Determine order of reaction by measuring the. It gives a relationship between the concentration of a solution and the attenuation of light as it. “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore. Beer Lambert Law Lab.
From www.youtube.com
Spectrophotometry and Beer's Law YouTube Beer Lambert Law Lab It gives a relationship between the concentration of a solution and the attenuation of light as it. “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to. Beer Lambert Law Lab.
From studylib.net
BeerLambert Law lab reportedited Beer Lambert Law Lab The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). Determine order of reaction by measuring the. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a. Beer Lambert Law Lab.
From fyooddpjn.blob.core.windows.net
Beer Lambert Law Enzyme Activity at Russell Bingaman blog Beer Lambert Law Lab It gives a relationship between the concentration of a solution and the attenuation of light as it. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light. Explore. Beer Lambert Law Lab.
From www.youtube.com
Beer's Law Lab Procedure YouTube Beer Lambert Law Lab Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). The amount of light that a species absorbs in a spectroscopic transition can be related. Beer Lambert Law Lab.
From www.youtube.com
Spectrophotometry BeerLambert Law. YouTube Beer Lambert Law Lab “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). It gives a relationship between. Beer Lambert Law Lab.
From www.youtube.com
Beer's Law Overview YouTube Beer Lambert Law Lab It gives a relationship between the concentration of a solution and the attenuation of light as it. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light.. Beer Lambert Law Lab.
From fyooddpjn.blob.core.windows.net
Beer Lambert Law Enzyme Activity at Russell Bingaman blog Beer Lambert Law Lab “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. It gives a relationship between the concentration of a solution and the attenuation of light as it.. Beer Lambert Law Lab.
From chemdictionary.org
BeerLambert Law History, Definition & Example Calculation Beer Lambert Law Lab Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light. It gives a relationship between the concentration of a solution and the attenuation of light as it. Determine order of reaction. Beer Lambert Law Lab.
From www.pdfprof.com
loi de beer lambert absorbance Beer Lambert Law Lab Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). It gives a relationship between. Beer Lambert Law Lab.
From studylib.net
BeerLambert Absorption Law Beer Lambert Law Lab Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). It gives a relationship between the concentration of a solution and the attenuation of light as it. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance. Beer Lambert Law Lab.
From calculatorghw.blogspot.com
Beer Lambert Law Calculator CALCULATOR GHW Beer Lambert Law Lab The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). Determine order of reaction by measuring the. “the thicker the glass, the darker the brew, the less the light that passes through.”. Beer Lambert Law Lab.
From www.youtube.com
Beer Lambert's Law, Absorbance & Transmittance Spectrophotometry Beer Lambert Law Lab Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light. The amount of light. Beer Lambert Law Lab.
From oneclass.com
CHEM 126 Lecture 5 Experiment 5 Beer Lambert Law and Determination of Beer Lambert Law Lab It gives a relationship between the concentration of a solution and the attenuation of light as it. Determine order of reaction by measuring the. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore. Beer Lambert Law Lab.
From www.slideserve.com
PPT Absorption and Scattering PowerPoint Presentation, free download Beer Lambert Law Lab The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light. It gives a relationship between the concentration of a solution and the. Beer Lambert Law Lab.
From www.thoughtco.com
Beer's Law Definition and Equation Beer Lambert Law Lab Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). It gives a relationship between the concentration of a solution and the attenuation of light as it. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with. Beer Lambert Law Lab.
From www.youtube.com
Spectrophotometry Learn the BeerLambert law with absorbance Beer Lambert Law Lab “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Explore beer's law by creating. Beer Lambert Law Lab.
From gioicdiik.blob.core.windows.net
Beer Lambert Law Ppt at Jaimie Levell blog Beer Lambert Law Lab Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). The amount of light. Beer Lambert Law Lab.
From www.slideserve.com
PPT Spectrophotometric Determination of Iron Using 1,10 Beer Lambert Law Lab The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. It gives a relationship between. Beer Lambert Law Lab.
From www.youtube.com
Visible Spec BeerLambert Law Lab YouTube Beer Lambert Law Lab Determine order of reaction by measuring the. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light. Calculate the concentration of a. Beer Lambert Law Lab.