Emission Spectra Flame Test . Electrons in atoms can only exist at certain. For example, the line spectra shown below for the. To the naked eye, when an element is vaporized in a flame (or an electrical discharge)the emission spectrum will appear to be just one color. The result is called a line emission spectrum, and can serve as a ‘fingerprint’ of the element to which the atoms belong. The premise is that heat gives energy to elements and ions, causing them to emit light at a characteristic color or emission spectrum. Flame tests can be used to identify some metal ions (cations). Lithium, sodium, potassium, calcium and copper compounds produce distinctive colours in flame. The flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. The emission of light from any one element can be understood by considering how electrons move between discrete energy levels in atoms.
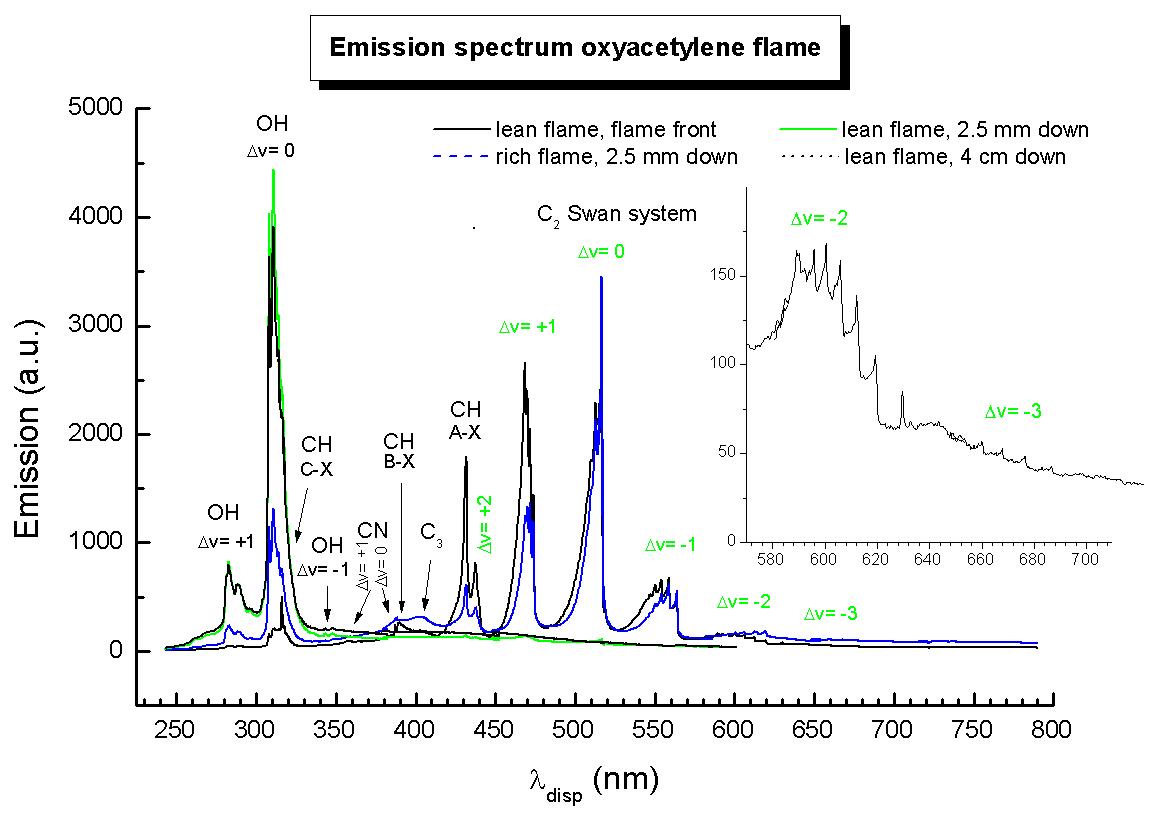
from rkdmb.home.xs4all.nl
Lithium, sodium, potassium, calcium and copper compounds produce distinctive colours in flame. The emission of light from any one element can be understood by considering how electrons move between discrete energy levels in atoms. Electrons in atoms can only exist at certain. The flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. Flame tests can be used to identify some metal ions (cations). The result is called a line emission spectrum, and can serve as a ‘fingerprint’ of the element to which the atoms belong. To the naked eye, when an element is vaporized in a flame (or an electrical discharge)the emission spectrum will appear to be just one color. The premise is that heat gives energy to elements and ions, causing them to emit light at a characteristic color or emission spectrum. For example, the line spectra shown below for the.
Flame spectroscopy
Emission Spectra Flame Test The result is called a line emission spectrum, and can serve as a ‘fingerprint’ of the element to which the atoms belong. The emission of light from any one element can be understood by considering how electrons move between discrete energy levels in atoms. Lithium, sodium, potassium, calcium and copper compounds produce distinctive colours in flame. The premise is that heat gives energy to elements and ions, causing them to emit light at a characteristic color or emission spectrum. The flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. Electrons in atoms can only exist at certain. For example, the line spectra shown below for the. Flame tests can be used to identify some metal ions (cations). The result is called a line emission spectrum, and can serve as a ‘fingerprint’ of the element to which the atoms belong. To the naked eye, when an element is vaporized in a flame (or an electrical discharge)the emission spectrum will appear to be just one color.
From rkdmb.home.xs4all.nl
Flame spectroscopy Emission Spectra Flame Test Lithium, sodium, potassium, calcium and copper compounds produce distinctive colours in flame. The emission of light from any one element can be understood by considering how electrons move between discrete energy levels in atoms. Flame tests can be used to identify some metal ions (cations). Electrons in atoms can only exist at certain. For example, the line spectra shown below. Emission Spectra Flame Test.
From studymind.co.uk
Flame Emission Spectroscopy (GCSE Chemistry) Study Mind Emission Spectra Flame Test The flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. Electrons in atoms can only exist at certain. Flame tests can be used to identify some metal ions (cations). For example, the line spectra shown below for the. The emission of light from any one element can be understood by considering. Emission Spectra Flame Test.
From www.savemyexams.com
Flame Emission Spectroscopy AQA GCSE Chemistry Revision Notes 2018 Emission Spectra Flame Test The emission of light from any one element can be understood by considering how electrons move between discrete energy levels in atoms. Flame tests can be used to identify some metal ions (cations). Electrons in atoms can only exist at certain. To the naked eye, when an element is vaporized in a flame (or an electrical discharge)the emission spectrum will. Emission Spectra Flame Test.
From studylib.net
Emission Spectrum Lab and Flame Test Discussion Questions Emission Spectra Flame Test The premise is that heat gives energy to elements and ions, causing them to emit light at a characteristic color or emission spectrum. The result is called a line emission spectrum, and can serve as a ‘fingerprint’ of the element to which the atoms belong. Electrons in atoms can only exist at certain. To the naked eye, when an element. Emission Spectra Flame Test.
From www.thoughtco.com
What Is Luminosity and What does it Tell Us? Emission Spectra Flame Test Flame tests can be used to identify some metal ions (cations). The result is called a line emission spectrum, and can serve as a ‘fingerprint’ of the element to which the atoms belong. For example, the line spectra shown below for the. The premise is that heat gives energy to elements and ions, causing them to emit light at a. Emission Spectra Flame Test.
From www.bbc.com
Chemical analysis Revision 2 National 5 Chemistry BBC Bitesize Emission Spectra Flame Test The emission of light from any one element can be understood by considering how electrons move between discrete energy levels in atoms. The premise is that heat gives energy to elements and ions, causing them to emit light at a characteristic color or emission spectrum. To the naked eye, when an element is vaporized in a flame (or an electrical. Emission Spectra Flame Test.
From www.youtube.com
Atomic Spectroscopy Flame Emission The Flame Test YouTube Emission Spectra Flame Test The premise is that heat gives energy to elements and ions, causing them to emit light at a characteristic color or emission spectrum. For example, the line spectra shown below for the. The flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. To the naked eye, when an element is vaporized. Emission Spectra Flame Test.
From www.researchgate.net
Emission spectra of different light sources (a) incandescent tungsten Emission Spectra Flame Test For example, the line spectra shown below for the. The premise is that heat gives energy to elements and ions, causing them to emit light at a characteristic color or emission spectrum. The emission of light from any one element can be understood by considering how electrons move between discrete energy levels in atoms. To the naked eye, when an. Emission Spectra Flame Test.
From electricaltestmodules.blogspot.com
Electrical test modules Flame tests and emission spectra Emission Spectra Flame Test The flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. Flame tests can be used to identify some metal ions (cations). The emission of light from any one element can be understood by considering how electrons move between discrete energy levels in atoms. The result is called a line emission spectrum,. Emission Spectra Flame Test.
From www.nagwa.com
Lesson Atomic Emission Spectroscopy Nagwa Emission Spectra Flame Test The result is called a line emission spectrum, and can serve as a ‘fingerprint’ of the element to which the atoms belong. Flame tests can be used to identify some metal ions (cations). The emission of light from any one element can be understood by considering how electrons move between discrete energy levels in atoms. Lithium, sodium, potassium, calcium and. Emission Spectra Flame Test.
From terrytao.wordpress.com
A second draft of a nontechnical article on universality What's new Emission Spectra Flame Test Lithium, sodium, potassium, calcium and copper compounds produce distinctive colours in flame. The emission of light from any one element can be understood by considering how electrons move between discrete energy levels in atoms. Flame tests can be used to identify some metal ions (cations). The result is called a line emission spectrum, and can serve as a ‘fingerprint’ of. Emission Spectra Flame Test.
From www.youtube.com
Flame Photometry Flame Photometer Atomic Emission Spectroscopy Emission Spectra Flame Test The result is called a line emission spectrum, and can serve as a ‘fingerprint’ of the element to which the atoms belong. Electrons in atoms can only exist at certain. The flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. For example, the line spectra shown below for the. To the. Emission Spectra Flame Test.
From www.chegg.com
Solved Part 2 Emission Spectra In the flame test you saw a Emission Spectra Flame Test The result is called a line emission spectrum, and can serve as a ‘fingerprint’ of the element to which the atoms belong. Flame tests can be used to identify some metal ions (cations). To the naked eye, when an element is vaporized in a flame (or an electrical discharge)the emission spectrum will appear to be just one color. The emission. Emission Spectra Flame Test.
From namrataheda.blogspot.com
B for Biology Spectrophotometry Flame Photometry Emission Spectra Flame Test The premise is that heat gives energy to elements and ions, causing them to emit light at a characteristic color or emission spectrum. Electrons in atoms can only exist at certain. The result is called a line emission spectrum, and can serve as a ‘fingerprint’ of the element to which the atoms belong. For example, the line spectra shown below. Emission Spectra Flame Test.
From studiousguy.com
Sodium (Na) Properties & Uses StudiousGuy Emission Spectra Flame Test Lithium, sodium, potassium, calcium and copper compounds produce distinctive colours in flame. The flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. To the naked eye, when an element is vaporized in a flame (or an electrical discharge)the emission spectrum will appear to be just one color. Flame tests can be. Emission Spectra Flame Test.
From www.chegg.com
Solved Lab 11 Flame Tests and Atomic Emission Spectra Emission Spectra Flame Test To the naked eye, when an element is vaporized in a flame (or an electrical discharge)the emission spectrum will appear to be just one color. The premise is that heat gives energy to elements and ions, causing them to emit light at a characteristic color or emission spectrum. Electrons in atoms can only exist at certain. For example, the line. Emission Spectra Flame Test.
From slidetodoc.com
ATOMIC SPECTRA Objectives 1 Determine the emission spectrum Emission Spectra Flame Test The flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. The premise is that heat gives energy to elements and ions, causing them to emit light at a characteristic color or emission spectrum. The emission of light from any one element can be understood by considering how electrons move between discrete. Emission Spectra Flame Test.
From socratic.org
Question 20ecb Socratic Emission Spectra Flame Test To the naked eye, when an element is vaporized in a flame (or an electrical discharge)the emission spectrum will appear to be just one color. Flame tests can be used to identify some metal ions (cations). The result is called a line emission spectrum, and can serve as a ‘fingerprint’ of the element to which the atoms belong. For example,. Emission Spectra Flame Test.
From www.youtube.com
2.2 Flame tests (SL) YouTube Emission Spectra Flame Test Electrons in atoms can only exist at certain. The flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. Flame tests can be used to identify some metal ions (cations). For example, the line spectra shown below for the. Lithium, sodium, potassium, calcium and copper compounds produce distinctive colours in flame. The. Emission Spectra Flame Test.
From sciencephotogallery.com
Flame Emission Spectra Of Alkali Metals by Science Photo Library Emission Spectra Flame Test To the naked eye, when an element is vaporized in a flame (or an electrical discharge)the emission spectrum will appear to be just one color. Lithium, sodium, potassium, calcium and copper compounds produce distinctive colours in flame. The emission of light from any one element can be understood by considering how electrons move between discrete energy levels in atoms. Flame. Emission Spectra Flame Test.
From www.youtube.com
Flame Atomic Absorption Spectroscopy Demonstration YouTube Emission Spectra Flame Test The flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. The premise is that heat gives energy to elements and ions, causing them to emit light at a characteristic color or emission spectrum. Electrons in atoms can only exist at certain. The emission of light from any one element can be. Emission Spectra Flame Test.
From ucscphysicsdemo.sites.ucsc.edu
Linear Spectra UCSC Physics Demonstration Room Emission Spectra Flame Test Electrons in atoms can only exist at certain. To the naked eye, when an element is vaporized in a flame (or an electrical discharge)the emission spectrum will appear to be just one color. Flame tests can be used to identify some metal ions (cations). The emission of light from any one element can be understood by considering how electrons move. Emission Spectra Flame Test.
From mohamednewscaldwell.blogspot.com
Determination of Sodium by Flame Emission Spectroscopy Emission Spectra Flame Test To the naked eye, when an element is vaporized in a flame (or an electrical discharge)the emission spectrum will appear to be just one color. The flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. The result is called a line emission spectrum, and can serve as a ‘fingerprint’ of the. Emission Spectra Flame Test.
From www.mrpalermo.com
Virtual Lab Flame Test & Spectroscopy Mr. Palermo's Flipped Emission Spectra Flame Test The result is called a line emission spectrum, and can serve as a ‘fingerprint’ of the element to which the atoms belong. The flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. Lithium, sodium, potassium, calcium and copper compounds produce distinctive colours in flame. Flame tests can be used to identify. Emission Spectra Flame Test.
From laderarctic.weebly.com
Emission spectra laderarctic Emission Spectra Flame Test Electrons in atoms can only exist at certain. The flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. For example, the line spectra shown below for the. The emission of light from any one element can be understood by considering how electrons move between discrete energy levels in atoms. To the. Emission Spectra Flame Test.
From www.pinterest.se
Infographic. flame test of elements based on spectral emission when Emission Spectra Flame Test Electrons in atoms can only exist at certain. The premise is that heat gives energy to elements and ions, causing them to emit light at a characteristic color or emission spectrum. Lithium, sodium, potassium, calcium and copper compounds produce distinctive colours in flame. Flame tests can be used to identify some metal ions (cations). For example, the line spectra shown. Emission Spectra Flame Test.
From www.youtube.com
Flame photometry/Flame Emission Spectroscopy (FES)/Atomic emission Emission Spectra Flame Test The premise is that heat gives energy to elements and ions, causing them to emit light at a characteristic color or emission spectrum. The emission of light from any one element can be understood by considering how electrons move between discrete energy levels in atoms. Electrons in atoms can only exist at certain. Lithium, sodium, potassium, calcium and copper compounds. Emission Spectra Flame Test.
From electricaltestmodules.blogspot.com
Electrical test modules Flame tests and emission spectra Emission Spectra Flame Test The premise is that heat gives energy to elements and ions, causing them to emit light at a characteristic color or emission spectrum. Lithium, sodium, potassium, calcium and copper compounds produce distinctive colours in flame. Electrons in atoms can only exist at certain. Flame tests can be used to identify some metal ions (cations). To the naked eye, when an. Emission Spectra Flame Test.
From studylib.net
Emission Spectra and Flame Tests Emission Spectra Flame Test Lithium, sodium, potassium, calcium and copper compounds produce distinctive colours in flame. The result is called a line emission spectrum, and can serve as a ‘fingerprint’ of the element to which the atoms belong. The emission of light from any one element can be understood by considering how electrons move between discrete energy levels in atoms. The premise is that. Emission Spectra Flame Test.
From mavink.com
Flame Emission Spectroscopy Diagram Emission Spectra Flame Test Flame tests can be used to identify some metal ions (cations). Lithium, sodium, potassium, calcium and copper compounds produce distinctive colours in flame. To the naked eye, when an element is vaporized in a flame (or an electrical discharge)the emission spectrum will appear to be just one color. For example, the line spectra shown below for the. The premise is. Emission Spectra Flame Test.
From www.slideserve.com
PPT Chemistry in Action PowerPoint Presentation, free download ID Emission Spectra Flame Test To the naked eye, when an element is vaporized in a flame (or an electrical discharge)the emission spectrum will appear to be just one color. The emission of light from any one element can be understood by considering how electrons move between discrete energy levels in atoms. The flame test is a qualitative test in analytical chemistry used to help. Emission Spectra Flame Test.
From www.degruyter.com
Taking flame tests one step forward the case of a DIY atomic emission Emission Spectra Flame Test The premise is that heat gives energy to elements and ions, causing them to emit light at a characteristic color or emission spectrum. The flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. For example, the line spectra shown below for the. To the naked eye, when an element is vaporized. Emission Spectra Flame Test.
From myans.bhantedhammika.net
Flame Test And Atomic Spectra Lab Answer Key Emission Spectra Flame Test To the naked eye, when an element is vaporized in a flame (or an electrical discharge)the emission spectrum will appear to be just one color. The premise is that heat gives energy to elements and ions, causing them to emit light at a characteristic color or emission spectrum. Flame tests can be used to identify some metal ions (cations). The. Emission Spectra Flame Test.
From sciencenotes.org
Flame Test Colors and Procedure (Chemistry) Emission Spectra Flame Test Flame tests can be used to identify some metal ions (cations). Lithium, sodium, potassium, calcium and copper compounds produce distinctive colours in flame. The emission of light from any one element can be understood by considering how electrons move between discrete energy levels in atoms. The result is called a line emission spectrum, and can serve as a ‘fingerprint’ of. Emission Spectra Flame Test.
From www.alamy.com
Iron chloride Stock Vector Images Alamy Emission Spectra Flame Test Flame tests can be used to identify some metal ions (cations). The emission of light from any one element can be understood by considering how electrons move between discrete energy levels in atoms. Lithium, sodium, potassium, calcium and copper compounds produce distinctive colours in flame. To the naked eye, when an element is vaporized in a flame (or an electrical. Emission Spectra Flame Test.